Figure 2. Schematic representation of the manufacture process of CAR-T cells used in clinical trials.
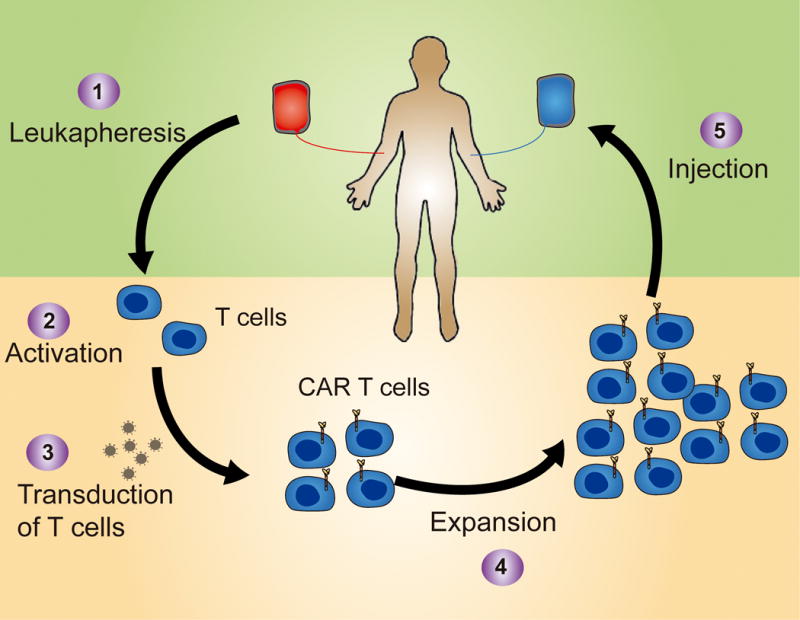
T cells are collected from the patient usually through leukapheresis (1) and then activated (2) and transduced with a retroviral vector (3). CAR-T cells are then expanded (4) to obtain sufficient numbers to infuse back into the patient a few days or weeks later (5).
