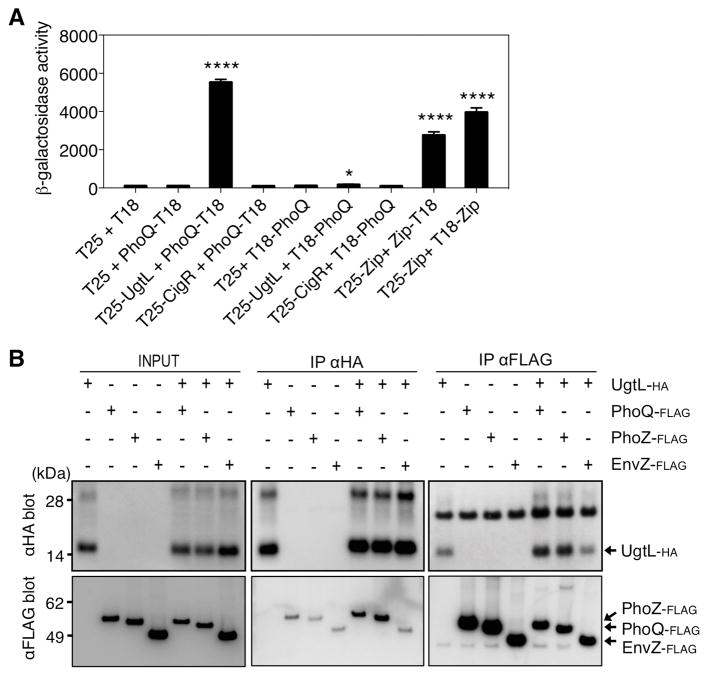
Fig. 2. The UgtL and PhoQ proteins interact.
(A). β-galactosidase activity in bacterial two-hybrid system assays in E. coli BTH101 expressing the indicated fusion proteins. In each experimental condition, the bacteria carried the two Bordetella pertussis adenylate cyclase fragments T25 and T18 either alone or fused to UgtL, CigR, or PhoQ in the indicated combinations. The adenylate cyclase fragment was fused to the N terminus in fusion proteins T25-UgtL, T25-CigR, and T18-PhoQ and to the C terminus in fusion protein PhoQ-T18. T25-Zip, Zip-T18, and T18-Zip were used as positive controls. The mean and SD from three independent experiments are shown. Unpaired students T-test were performed between strains harboring empty vectors with the other combinations; * p < 0.05, **** p <0.0001. (B) Pulldown assays showing interactions between in vitro–synthesized UgtL-HA, PhoQ-FLAG, PhoZ-FLAG, and EnvZ-FLAG proteins. Samples were analyzed by Western blotting using antibodies recognizing the HA epitope and the FLAG epitope. Densitometry of each blot with ImageJ software is shown below the blots in the same order using arbitrary units (AU). Dashed lines in the densitometry graphs indicate signals from nonspecific binding of the UgtL-HA and FLAG-tagged proteins to antibodies recognizing the FLAG and HA epitopes, respectively. The data are representative of two independent experiments, which produced similar results.