Figure 6.
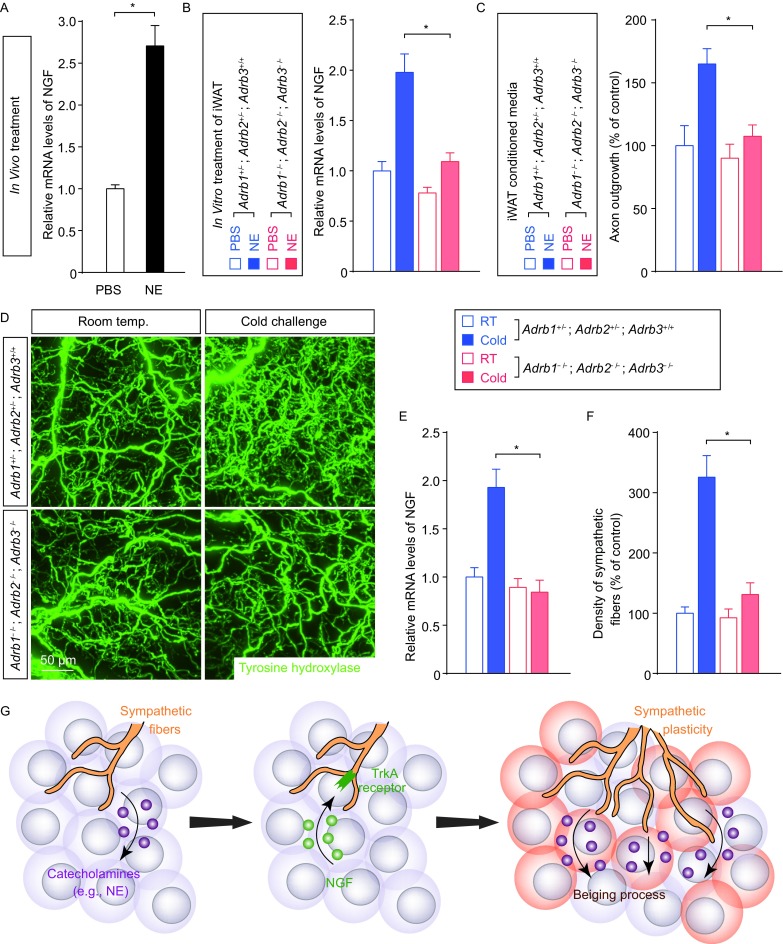
Cold-elicited NGF expression in WAT depends on the catecholamine signal. (A) The wildtype mice maintained at room temperature were treated with norepinephrine (NE) or vehicle control. Expression levels of NGF in iWAT were determined by the qPCR analysis. n = 5, mean ± SEM, *P < 0.01. (B and C) iWAT of Adrb1−/−; Adrb2−/−; Adrb3−/−mice or control mice (Adrb1+/−; Adrb2+/−; Adrb3+/+) was in vitro treated with 100 μmol/L norepinephrine (NE) or control PBS. (B) Expression levels of NGF in iWAT were determined by the qPCR analysis. n = 4, mean ± SEM, *P < 0.01. (C) The conditioned media of iWAT were collected and administrated onto cultured sympathetic neurons. Lengths of the axon outgrowth were quantified. n = 4, mean ± SEM, *P < 0.01. (D–F) Adrb1−/−; Adrb2−/−; Adrb3−/− mice or control mice (Adrb1+/−; Adrb2+/−; Adrb3+/+) were subject to cold challenge. (D) iWAT was processed for the volume fluorescence-imaging of anti-tyrosine hydroxylase. Representative 3D projections of iWAT imaged at 12.6× magnification on the lightsheet microscope. (E) Expression levels of NGF in iWAT were determined by the qPCR analysis. n = 4, mean ± SEM, *P < 0.01. (F) Density of the sympathetic fibers in iWAT was quantified. n = 4, mean ± SEM, *P < 0.01. (G) Diagram of the key involvement of intra-adipose sympathetic plasticity regulated by the NGF-TrkA signal in the cold-induced beiging process of WAT
