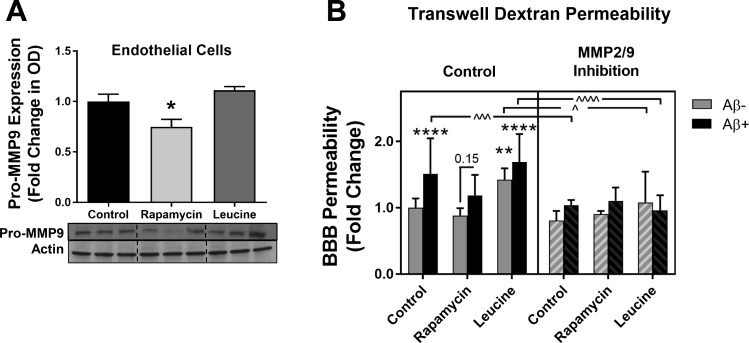
Fig. 5.
Matrix metalloproteinase (MMP)-9 activity is implicated in amyloid-β (Aβ)- and leucine-induced in vitro blood-brain barrier (BBB) breakdown. A: rapamycin-induced attenuation of mammalian/mechanistic target of rapamycin (mTOR) decreases pro-MMP-9 expression in cultured endothelial cells. OD, optical density. *Significant decrease vs. control [q(15) = 3.95, P = 0.034] by Tukey’s post hoc test on a significant one-way ANOVA [F(2,15) = 8.58, P = 0.003]. Values are means ± SE; n = 6 mice/condition. B: MMP-9 mediates Aβ-induced BBB breakdown in vitro as assessed by FITC-dextran leakage. Three-way ANOVA revealed significant main effects: MMP-9 [F(1,168) = 50.5, P < 0.0001], Aβ [F(1,168) = 30.2, P < 0.0001], and mTOR [F(2,168) = 14.6, P < 0.0001] activity. Holm-Sidak’s multiple-comparison post hoc test revealed that Aβ induced BBB permeability in control cells (****t = 4.96, P < 0.0001). mTOR overactivation with leucine was sufficient to induce barrier disruption (**t = 4.1, P = 0.0029) and exacerbate Aβ-induced barrier permeability (****t = 6.67, P < 0.0001). Attenuation of mTOR with rapamycin treatment prevented Aβ-induced disruption of barrier function (t = 2.92, P = 0.15). Furthermore, MMP-2/MMP-9 inhibition prevented barrier disruption by Aβ in control cells (^^^t = 4.58, P = 0.0005), barrier permeability induced by mTOR overactivation in leucine-treated cells (^t = 3.33, P = 0.044), and exacerbation of barrier permeability by Aβ in the presence of hyperactive mTOR (^^^^t = 7.06, P < 0.0001). Data were normalized to the control group and are expressed as fold change. Values are means ± SE; n = 15 mice/condition.