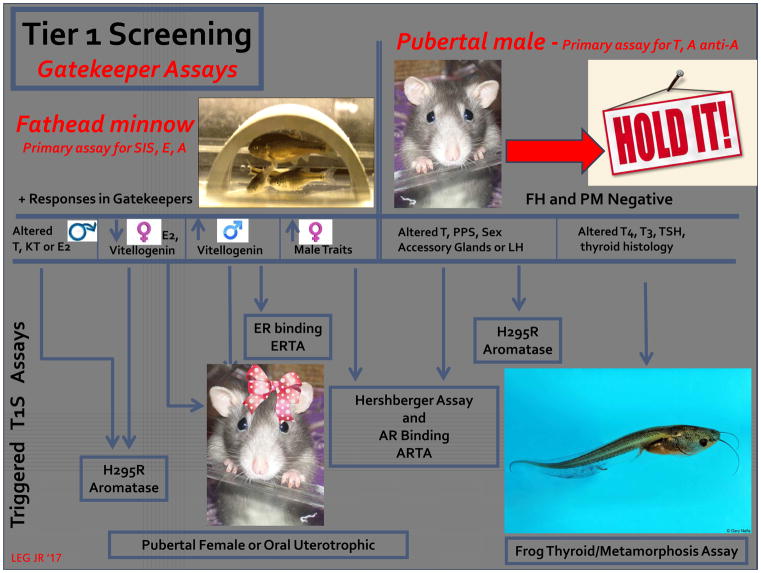
Figure 2.
A potential approach to streamlining the eleven EPA, EDSP Tier 1 Screening assays based upon a “weight of evidence” review of the literature on the effects of chemicals in the test guideline protocols [4; 18]. In this approach, initially two “gatekeeper” assays, the fathead minnow short-term reproductive test and the pubertal male rat assay, are conducted. If these are negative, then no other screening is necessary. This relies upon the fish assay for the detection of estrogens, and chemicals that inhibit steroidogenesis. Androgens and antiandrogens and chemicals that alter thyroid function are detected in the pubertal male assay. If either gatekeeper assay is positive (in the absence of overt toxicity) then additional screening is triggered on a mode-of-action basis [4; 16; 18]. (abbreviations: T1S- EPA, EDSP tier 1 screening assay battery; AR – androgen receptor; ARTA- androgen receptor transcriptional antagonist assay; T – testosterone; PPS – preputial separation; LH – luteinizing hormone; T4 – thyroxine; T3 – triiodothyronine; TSH – thyroid stimulating hormone; FH – fathead minnow reproduction test; PM – pubertal male assay; E2 – 17 beta estradiol; KT – ketotestosterone; H295R – cell line used to assess steroidogenesis in vitro.