Figure 3.
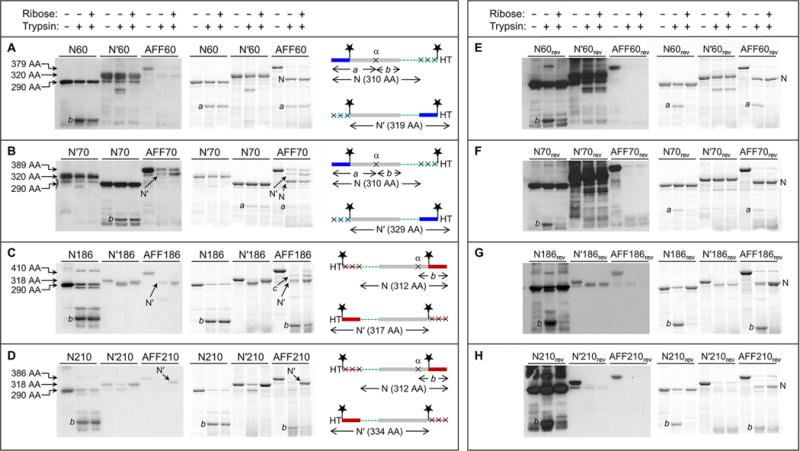
Fold shift test by partial trypsin digestion. Gels at the left are anti-HT Western blots. Gels at the right are BODIPY fluorescence scans of the same gels at the left. Amino acid sequences are shown at the far right, schematized to highlight the N and N′ conformations and the positions of the HTs, BODIPY groups (stars), and trypsin cleavage sites (×). The same color scheme as in Figure 1 is used, as well as same the rectangle and line designation for folded and unfolded structure, respectively. Amino acid lengths of undigested N analogues, N′ analogues, and AFF constructs are labeled to the left of the gels. These lengths, as well as the amino acid sequence diagrams, are omitted from panels E–H because they are identical to those in panels A–D, respectively. Note that the loading order of N70 and N′70 is switched compared to that of gels of the other proteins.
