Fig. 4.
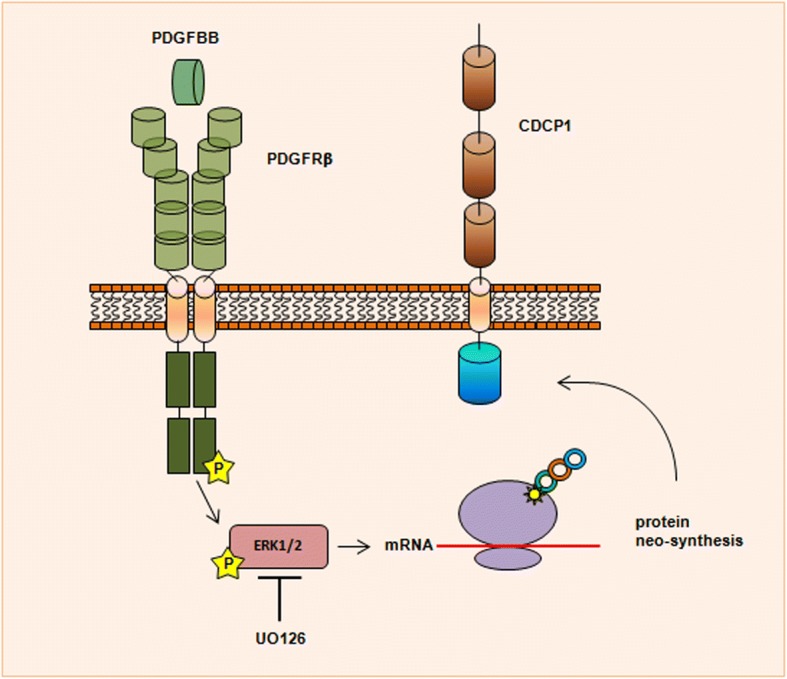
Schematic representation of CDCP1 upregulation induced by PDGF-BB/PDGFRβ pathway through ERK1/2 activation. PDGFRβ dimerizes and is activated upon binding of the PDGF-BB ligand, causing the activation of the kinase domain, visualized as tyrosine phosphorylation (P) of the receptor molecules. In conjunction with dimerization and kinase activation, the receptor molecules undergoes a conformational changes, which allow a basal kinase activity, leading to full enzymatic activity directed toward downstream mediators such as ERK1/2. ERK1/2 activity is necessary for CDCP1 protein neo-synthesis, as demonstrated by the reduction of CDCP1 protein levels in presence of UO126, an inhibitor of ERK1/2
