Abstract
Triage of posterior circulation stroke from emergent large-vessel occlusion (pc-ELVO) is challenging owing to the stuttering clinical course and potential for rapid decline. Growing clinical data support the use of mechanical thrombectomy in pc-ELVO, but there are limited data addressing the clinical and imaging criteria for patient selection. We present our triage algorithm used to select patients for endovascular therapy (EVT) in the setting of pc-ELVOS. We use a consecutive retrospective database from 2004 to 2016 to describe the practice patterns and prognostic factors for pc-ELVO patients treated using both medical and EVT. Patients with moderate to severe deficits (NIHSS > 10) did better when they received EVT (p < 0.03), whereas patients with stable, mild deficits (NIHSS ≤ 10) did well (90% favorable outcome) regardless of treatment type. Roughly one-third of patients presenting with mild deficits deteriorated to moderate to severe deficits (NIHSS > 10), most of whom subsequently received EVT (9 of 12), with 56% favorable outcomes. Cerebellar and brainstem infarct volumes were independent imaging predictors of outcome. These results can be used to define triage criteria for use of EVT in pc-ELVO in future practice and clinical trials.
Keywords: Basilar occlusion, large-vessel occlusion, mechanical thrombectomy, posterior circulation, stroke
Introduction
Posterior circulation stroke from emergent large-vessel occlusion (pc-ELVO) is difficult to treat and has high mortality and morbidity, historically approaching 80%.1 Endovascular therapy (EVT), including mechanical thrombectomy and aspiration, has been used cautiously in the posterior circulation since 2009, when the Basilar Artery International Cooperation Study (BASICS), a large multicenter prospective registry, was unable to show a significant benefit of EVT over medical management.2 Although practice patterns vary, EVT has generally been reserved for severe posterior circulation strokes. Landmark trials in 2015 and 2016 demonstrated the efficacy of EVT for LVO in anterior circulation stroke.3–9 Since those studies, there has been renewed effort to demonstrate a similar effect for EVT in the posterior circulation. However, the relatively low incidence and the varied presentation of posterior LVO infarcts may preclude a large randomized prospective trial. In the last few years, case series and retrospective trials have provided preliminary evidence that EVT may provide benefit in posterior circulation LVO.10–12
It remains unclear which pc-ELVO patients may benefit from intervention. This is difficult to address partly because of the variation in presentation and the stuttering course of this disease. Patients often present with relatively low National Institutes of Health Stroke Scale (NIHSS) and then precipitously decline. Prior retrospective studies included a broad range of stroke severity, with average NIHSS ranging from 10 to 24. Furthermore, only one of these papers compares EVT patients with medically treated patients at the same institution. To develop a proper algorithm and understand thresholds for treatment, it is important to assess cohorts including medical and EVT patients.
We approach these issues by studying a consecutive retrospective cohort of patients presenting to our institution with pc-ELVO over a 12-year span, including medical and EVT patients. We describe the practice patterns and trends that evolved over those years and identify which patients benefited from these therapies. We define clinical and imaging criteria based on these empirical data to triage patients to medical or endovascular treatment.
Materials and methods
We generated a retrospective cohort of posterior circulation LVO patients by searching imaging and procedure databases at our institution using keywords including “basilar thrombus,” “basilar occlusion,” “basilar and infarct,” and “pontine infarct,” which yielded 242 patients from 1995 to 2016. Archived digital subtraction angiography was available for cases performed after 2003, so only patients treated from 2004 to 2016 were included. Inclusion criteria were: (a) angiographic evidence of vertebrobasilar occlusion and (b) imaging evidence of infarct (either computed tomography (CT) or magnetic resonance imaging (MRI)). Exclusion criteria included: (a) patients with nonocclusive thrombus or (b) anterior circulation infarcts (in addition to posterior circulation). Of the 242 total patients, 89 from 2004 to 2016 met these criteria. The study was conducted with hospital institutional review board approval.
Patients were dichotomized by modified Rankin Scale score (mRS) to favorable (mRS ≤ 2) and poor outcome (mRS > 2). Best clinical outcome was recorded at discharge or 90-day follow-up if available. Clinical characteristics compared between the two groups included age, gender, etiology (if discernable from medical record or imaging data), presenting NIHSS (p-NIHSS), most severe NIHSS prior to intervention (worst NIHSS, or w-NIHSS), hemorrhagic conversion (determined from susceptibility-weighted sequences or hyperattenuation on noncontrast CT), and treatment modality (medical therapy or EVT). In patients with limited exam, for example, those arriving to our institution intubated, NIHSS was estimated at 25.
Imaging characteristics included infarct volume, posterior Alberta Stroke Program Early CT Score (pc-ASPECTS), thrombus location and length, and for patients treated with EVT, the Thrombolysis in Cerebral Infarction score (TICI).13 The pc-ASPECTS scores were taken from pretreatment diffusion-weighted imaging (DWI) or CT/computed tomography angiography (CTA) in the absence of MRI. Infarct volume was estimated using the ABC/2 ellipsoid model from pretreatment DWI sequences;14 volumes from the cerebellum and brainstem were measured separately. The pc-ASPECTS was determined from the DWI sequences.15 Thrombus location was determined from CTA images and classified as involvement of the vertebral arteries, proximal, mid-, or distal basilar artery, and P1 segments. If thrombus spanned multiple segments, all involved segments were counted. Thrombus length was estimated using maximum intensity pixel (MIP) reformats from CTA images when available.
Categorical variables were compared using chi-squared and the Fisher exact test in the case of small n; continuous variables were compared using two-sided t-test. Logistic regression was used to model the effects of characteristics on outcomes. All statistical calculations were performed using R.16
Results
Demographics
We identified 89 patients treated at our institution from 2004 to 2016 for posterior circulation ischemia due to acute LVO (Table 1). Seventy percent (n = 62) of the patients were initially evaluated at an outside hospital and then transferred to Massachusetts General Hospital (MGH). The average time from ictus to arrival at the emergency room was 8.2 h: 4.3 h for patients brought directly by emergency medical services and 9.8 h for patients transferred from an outside institution. All-cause mortality at 90 days was 36%. Overall, 40% (n = 36) of the patients had a favorable outcome (mRS ≤ 2 at discharge or 90 days). The 90-day follow-up evaluations were available for 90% (n = 80) of the patients. There was no difference in outcome between patients transferred and those admitted directly. There was one major procedural complication resulting in patient death, a basilar artery perforation related to microcatheter and wire loop technique.
Table 1.
Clinical and imaging characteristics of patients with favorable (mRS ≤ 2) and poor (mRS > 2) outcomes.
| Clinical characteristics | mRS ≤ 2 | mRS > 2 | p value |
|---|---|---|---|
| Total | 36 (40%) | 53 (60%) | |
| Age (average) | 62.1 | 65.3 | 0.3 |
| Gender | |||
| Male | 21 (58%) | 36 (69%) | |
| Female | 15 (42%) | 17 (31%) | 0.5 |
| Transfer | |||
| Transfer from outside institution | 27 (75%) | 35 (66%) | 0.5 |
| Hours from ictus (average) | 7.3 | 8.8 | 0.3 |
| Stroke severity | |||
| NIHSS, presentation (average) | 11.0 | 17.8 | 0.0005 |
| NIHSS, worst (average) | 13.1 | 20.6 | 0.00003 |
| Deterioration after presentation | 11 (31%) | 21 (40%) | 0.7 |
| Treatment | |||
| IV heparin | 32 (89%) | 34 (64%) | 0.02 |
| IV tPA | 5 (14%) | 10 (19%) | 0.7 |
| EVT | 17 (47%) | 29 (55%) | 0.6 |
| ADAPT or stentriever | 6 (17%) | 7 (13%) | 0.8 |
| Imaging characteristics | |||
| pc-ASPECTS (average) | 7.4 | 5.4 | 0.000002 |
| Brainstem infarct (average, cc) | 0.8 | 2.3 | 0.0002 |
| Cerebellum infarct (average, cc) | 1.2 | 10.7 | 0.0002 |
| Hemorrhage | 4 (11%) | 14 (26%) | 0.13 |
| Clot length (cm) | 16.1 | 19.0 | 0.4 |
| Site of occlusion | |||
| Vertebral | 5 (14%) | 14 (26%) | 0.2 |
| Proximal basilar | 16 (44%) | 21 (39%) | 0.8 |
| Mid-basilar | 20 (56%) | 35 (66%) | 0.4 |
| Distal basilar | 23 (64%) | 43 (81%) | 0.1 |
| P1 | 9 (25%) | 11 (21%) | 0.8 |
P values calculated using Chi squared for conditional variables and two-sided t-test for continuous variables. Values < 0.05 are shown in bold. mRS: modified Rankin Scale; NIHSS: National Institutes of Health Stroke Scale; IV: intravenous; tPA: tissue plasminogen activator; EVT: endovascular therapy; ADAPT: a direct aspiration, first-pass technique for the endovascular treatment of stroke; pc-ASPECTS: posterior Alberta Stroke Program Early CT Score.
Major etiologies included atrial fibrillation (25%, n = 22), intracranial atherosclerotic disease (20%, n = 18), vertebral artery dissection (10%, n = 9), septic embolus (2%, n = 2), and patent foramen ovale (1%, n = 1). In roughly half of the cases (43/89), no cause was identified from chart review. Seven patients with a sole vertebral artery supplying the basilar artery were included in the cohort.
Imaging characteristics
Most patients were assessed initially with CTA (93%) and the remaining patients were evaluated with magnetic resonance angiography (MRA). Most patients additionally had MRI on admission (83%) and 33 of 46 patients (72%) had MRI prior to EVT. Poor outcome was associated with low pc-ASPECTS score and higher infarct volumes in the brainstem and cerebellum (Table 1), although only brainstem (p < 0.007) and cerebellar (p < 0.02) infarct volumes were independent predictors of outcome when these imaging characteristics were considered together in multivariate regression. Only cerebellar infarct volume was an independent predictor if NIHSS was included in the model. All patients with bilateral thalamic infarcts had poor outcome (n = 8, p < 0.02).
Treatment methods
Patients were treated using a variety of strategies. Most received intravenous (IV) heparin administration (74%) and of these just over half (n = 36) went on to receive EVT. Heparin administration was associated with favorable outcome (p < 0.02), but was primarily given to patients with mild and moderate strokes (average w-NIHSS 16.1 compared to 21.8, p < 0.002). It was not associated with outcome when controlling for stroke severity (w-NIHSS). Fifteen patients received IV tissue plasminogen activator (tPA) (17%) and about half went on to receive EVT. Eight medically treated patients did not receive heparin or IV tPA.
Fifty-two percent of the patients received some type of EVT. TICI 2B or 3 recanalization was achieved in 31 of 46 patients (67%). Since the study spanned 12 years, many of the EVT cases utilized older techniques including intra-arterial urokinase, wire manipulation, MERCI Concentric device and Penumbra separator device. Current devices, including a direct aspiration, first-pass technique for the endovascular treatment of stroke (ADAPT) and/or stent-retrievers, were utilized in 13 patients, of whom seven had a favorable outcome at 90 days, compared with 10 of 33 patients who received earlier types of EVT (p < 0.3).
Deterioration after presentation
Not uncommonly, patients presented with mild strokes and then deteriorated. We defined deterioration as a progressive decline in NIHSS > 2 from presentation. Approximately one-third of the patients (32/89) had clinical deterioration either during the initial evaluation at an outside hospital or while at our institution. Patients who deteriorated were no more likely to receive EVT (19/32 compared with 27/57, p = 0.4) and had similar outcomes compared to stable or improving patients (11/32 compared with 25/57 had good outcome, p = 0.5). Of those who deteriorated and were treated with EVT, 8 of 19 had good outcomes, compared with 3 of 13 who deteriorated but did not receive EVT (p = 0.5). Seven patients improved, with average NIHSS dropping from 10.4 on admission to 6.1. None of the improving patients received EVT and five of seven had favorable outcome.
Discussion
Acute stroke caused by posterior circulation LVO is challenging to treat and carries a high mortality. This study provides an extended view of treatment and outcomes in pc-ELVO and the empirical algorithm developed at our institution. Overall, 40% (n = 36) of our patients with pc-ELVO had a favorable outcome (mRS ≤ 2), with a mortality rate of 36% (n = 32). This is comparable to several recent studies10–12,17,18 and BASICS2 (Table 2).
Table 2.
Comparison of posterior circulation endovascular therapy (EVT) stroke studies.
| Study/author name | Treatment | N | NIHSS (average) | Favorable outcome |
|---|---|---|---|---|
| BASICS | EVT (IVT 14%) | 288 | 25 | 84 (29%)a |
| 2009, prospective | IVT | 121 | 21 | 50 (41%)a |
| AT | 183 | 15 | 66 (35%)a | |
| Andersson et al. | EVT | 27 | NA | 16 (57%) |
| 2013, retrospective | ||||
| Strbian et al. | IVT (EVT 5%) | 184 | 20 | 69 (36%) |
| 2013, prospective | ||||
| Yoon et al.10 | EVT (IVT 14%) | 50 | 11 | 27 (54%) |
| 2015, retrospective | ||||
| ENDOSTROKE | EVT (IVT 59%) | 148 | 20 | 50 (34%) |
| 2015, retrospective | ||||
| van Houwelingen et al. | EVT (IVT 100%) | 38 | 24 | 14 (37%) |
| 2016, retrospective | ||||
| Gilberti et al. | EVT (IVT 1%) | 32 | 13 | 13 (41%) |
| 2016, retrospective | ||||
| MGH 2017, retrospective | EVT (Hep 78%, IVT 17%) | 46 | 21 | 17 (37%) |
| Medical (Hep 70%, IVT 16%) | 43 | 14 | 19 (44%) |
Favorable outcome was defined as modified Rankin Scale (mRS) ≤ 2, except for BASICS, which used mRS 0–3 (indicated by a). BASICS: Basilar Artery International Cooperation Study; AT: arterial therapy; NIHSS: National Institutes of Health Stroke Scale; IVT: intravenous tissue plasminogen activator; Hep: heparin infusion; MGH: Massachusetts General Hospital.
There is large variability in the treatment strategies and outcome rates across these studies. One pattern that emerges is gradually better outcomes in more recent studies, which some authors have attributed to latest-generation stentrievers and aspiration catheters. We did not see a statistical difference in outcome between older and latest-generation endovascular techniques; however, our numbers were small (13 treated with ADAPT or stent-retrievers).
Triage criteria: Stroke severity
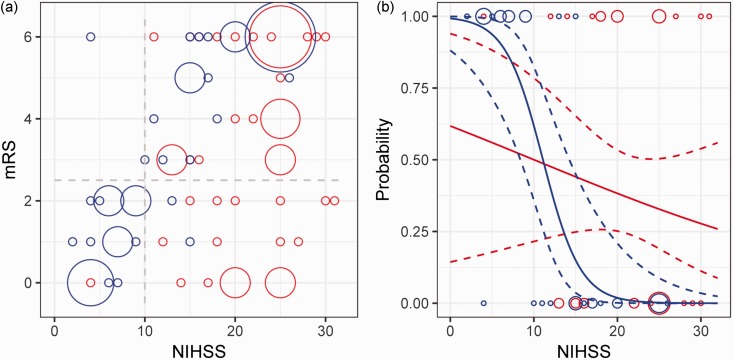
Stroke severity, assessed using the w-NIHSS, was an independent predictor of outcome in our series. However, in general, patients with higher w-NIHSS were more likely to be triaged to EVT (NIHSS 21.4 compared to 13.5, p < 0.000002). In subgroup analysis, patients with moderate to severe strokes (w-NIHSS > 10) treated by EVT had better outcomes than those treated medically (p < 0.03, Figure 1). When the probability for good outcome is modeled using the w-NIHSS, the EVT group had higher probability for good outcome relative to the medical group for all NIHSS > 10 (Figure 1). These findings support the recommendation of EVT for pc-ELVO patients with NIHSS > 10.
Figure 1.
Relationship of NIHSS and outcome. (a) Bubble plot of mRS and worst NIHSS for patients receiving medical therapy (blue) or endovascular therapy (red). The area of the circles is proportional to the number of patients at that point. Dashed gray lines delineate the boundaries between subgroups: favorable versus poor outcomes (mRS ≤ 2, mRS > 2), and mild versus moderate to severe strokes (NIHSS ≤ 10, NIHSS > 10). Patients in the moderate to severe stroke category did better if they received EVT (right lower quadrant, p < 0.03). (b) Probability of favorable outcome with NIHSS for medical (blue line) and EVT (red line) patients was calculated using logistic regression. Dotted lines are 95% confidence intervals. Patient data points used in the model are shown as circles along the top (p = 1, favorable outcome) and bottom (p = 0, poor outcome) axes, with area of each circle proportional to the number of patients. The models intersect at NIHSS = 11.2. NIHSS: National Institutes of Health Stroke Scale; mRS: modified Rankin Scale; EVT: endovascular therapy.
Triage criteria: Time from ictus
Patients arrived at our institution from 1 to 39 h after ictus (mean eight hours). No patients receiving EVT after 22 h had good outcome and the modeled probability for good outcome after EVT fell below 10% at 22 h (data not shown). This supports our institutional standard of offering EVT up to 24 h after ictus.
Many patients (32 of 89) presented with NIHSS ≤ 10, and in general, patients with mild, stable deficits did well with medical treatment only. For example, 17 of 19 patients (89%) with w-NIHSS ≤ 10 treated medically had favorable outcomes (see Figure 1). This serves as a counterpoint to a few recent retrospective series describing high rates of favorable ( > 50%) outcome after EVT in patients with relatively mild strokes (average NIHSS 13).10,17 However, patients presenting with mild pc-ELVO may progress to moderate or severe NIHSS. In our series, 12 patients (38%) with mild initial presentation progressed to NIHSS > 10. Most of these patients (9 of 12) received EVT and five of nine had favorable outcomes (p = 0.058). Given the high rates of favorable outcome in patients with mild pc-ELVO treated medically, we favor neuro-intensive care unit (ICU) care and heparin for pc-ELVO patients who are stable with NIHSS ≤ 10. Patients who rapidly deteriorate to NIHSS > 10 warrant immediate revascularization.
We observed seven patients who improved clinically on medical therapy alone, with average initial NIHSS of 10.4 improving to an average of 6.1. These patients improved rapidly on IV tPA or heparin, presumably owing to recanalization of short segment occlusions. None of these patients were treated with EVT and five of seven had favorable outcomes. Although it is impossible to draw conclusions on such small numbers of patients, it may be reasonable to forego EVT in patients who are rapidly improving after IV tPA or heparin therapy alone.
Triage criteria: Imaging characteristics
Although many other studies have demonstrated an association between pc-ASPECTS and infarct volume with outcome,12,17,19–21 only infarct volumes were independent imaging predictors of outcome in our series. All patients with bilateral thalamic infarcts had poor outcomes (n = 8, p < 0.02). We did see a trend toward increased average clot length in the poor outcome group (19.0 compared to 16.1 cm), which was not statistically significant as has been reported elsewhere.17,22 Similarly, we did not find that involvement of any segment of the vertebrobasilar system was associated with outcome, although a prior study showed patients with thrombus only at the distal basilar had more favorable outcomes.23
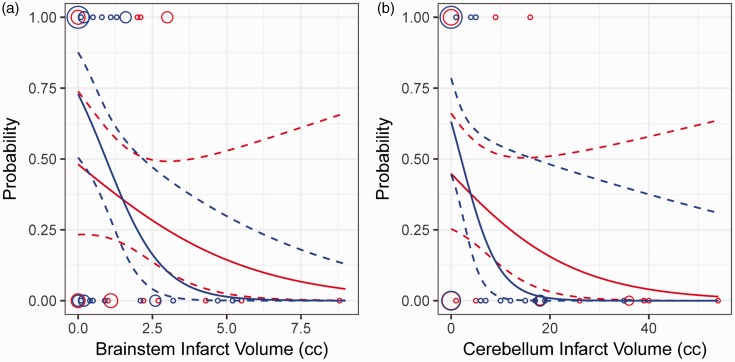
Determining a cut-off infarct volume for posterior circulation infarcts comparable to the 75–100 cc cut-off currently used in the anterior circulation is limited by the variety of infarct locations and the relative importance of highly eloquent brainstem regions compared to the cerebellum. In our cohort, the probability of good outcome was higher in EVT patients for cerebellar infarct volume > 4 cc, although no patients with cerebellar infarct volume > 20 cc had favorable outcomes (Figure 2(a)). No patients with brainstem infarct volumes > 3 cc had good outcomes (Figure 2(b)). Any infarct involving greater than half of the pons or midbrain is greater than 3 cc, and this concept is incorporated into our triage algorithm.
Figure 2.
Relationship of infarct volume and outcome. The probability of favorable outcome was calculated using logistic regression for medical and endovascular therapy patients based on brainstem (a) and cerebellum (b) infarct volumes. Dotted lines are 95% confidence intervals. Patient data points used in the model are shown as circles along the top (p = 1, favorable outcome) and bottom (p = 0, poor outcome) axes, with area of each circle proportional to the number of patients.
Treatment algorithm
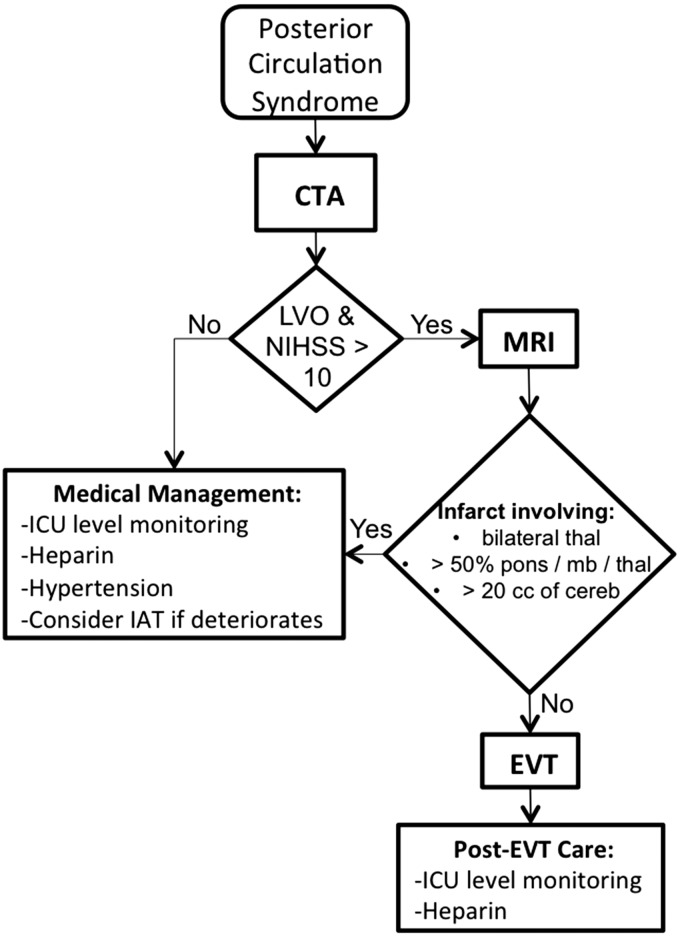
Given the technical challenges of EVT in the posterior circulation and the high morbidity and mortality of the disease, the decision to use EVT for acute vertebrobasilar occlusion is approached cautiously. Over the past 12 years, we have developed and revised a series of guidelines to standardize decision making across multiple providers. Although our institutional guidelines have evolved, several practice patterns emerge from review of this period. In general, we treated patients with severe strokes transferred from outside institutions. These patients were initially triaged with CTA and/or MRA, were started on heparin, and then went to EVT primarily based on stroke severity as assessed by NIHSS. Our empirical management algorithm is summarized in Figure 3.
Figure 3.
Treatment algorithm for acute ischemia in the setting of posterior circulation large-vessel occlusion. CTA: computed tomography angiography; LVO: large-vessel occlusion; NIHSS: National Institutes of Health Stroke Scale; MRI: magnetic resonance imaging; ICU: intensive care unit; IAT: intra-arterial therapy; EVT: endovascular therapy; thal: thalami; mb: midbrain; cereb: cerebellum.
We consider EVT for any patients presenting within 24 h from ictus, which can be extended for patients who initially have mild symptoms and then acutely deteriorate after 24 h. Approximately 80% (n = 71) of patients arrived at MGH within 12 h of ictus, and there was no significant difference in outcome between those who arrived before or after 12 h in this cohort.
Initial triage is based on the NIHSS and the finding of LVO on CTA. After arrival, the patient is transported as quickly as possible to the radiology department to facilitate rapid imaging acquisition. If LVO is demonstrated by CTA, the patient may be transferred immediately to the adjacent MRI scanner to perform a short, ischemia-tailored protocol including DWI. We prefer DWI to determine infarct volume in pc-ELVO because CT estimation is severely limited by extensive beam-hardening artifact in the posterior fossa. Other advanced imaging techniques such as perfusion or collateral circulation may be similarly helpful to identify pc-ELVO patients who may benefit from EVT.
Patients with infarcts involving greater than 20 cc of the cerebellum or greater than 50% of the pons or midbrain are less likely to benefit from EVT. These criteria are based on the observation that no patients in our cohort had favorable outcome under such circumstances. Establishing an upper bound or cut-off for infarct volume will require a larger cohort of EVT patients with expansion of enrollment criteria.
Patients with LVO on CTA but low NIHSS (<10) are admitted to the neuro-ICU for maximal medical management. The ICU setting is ideal for close clinical monitoring, blood pressure control, and initiation of anticoagulation, usually a heparin infusion. Frequent neurological examination allows rapid intervention in the event of deterioration, which occurs in more than 30% of patients.
Although the practice of anticoagulation remains controversial in the treatment acute ischemic stroke, we and others advocate the use of anticoagulation in pc-ELVO.24,25 The argument for anticoagulation in pc-ELVO is that even small clot aggregates can obstruct basilar perforators and reduce perfusion to vital brainstem centers. Heparin was used for most patients in our cohort (66 of 89 patients) and was associated with favorable outcome (p < 0.02). Heparin therapy may be withheld for a number of reasons including prior anticoagulation, hemorrhagic conversion, and when patients are made comfort measures only. The timing of anticoagulation relative to EVT may warrant further study.
Considerations specific to the posterior circulation
There are several factors that make acute stroke in the posterior circulation different from the anterior circulation. The anatomy of the posterior circulation includes a series of perforator arteries over its entire length from the medulla to the pons and thalami, which control basic consciousness, lower cranial nerves, and entire white matter tracts for motor and sensory. Patients may suffer bilateral loss of function compared to anterior circulation stroke affecting unilateral perforator arteries to the basal ganglia. However, reversible DWI in the pons can occur after stroke rescue26 and some patients with extensive DWI abnormality in the pons may gain functional recovery following intervention.27 This may suggest a different tissue tolerance for ischemia.
Second, the mix of disease states in the posterior circulation may be different with fewer cardioembolic cases and more dissections and intracranial atherosclerosis than in anterior LVO cohorts. Although there was no clear difference in outcomes between embolic and intracranial cerebral artery disease (ICAD) patients in our patients, a recent study suggests that ICAD patients fare more poorly after EVT than embolic patients.28 Third, the posterior circulation is more prone to procedural complications compared to the carotid artery because of tortuosity, multiple fixation points at the foramina transversaria and smaller vessel size. One may have to traverse a smaller vertebral artery to access the site of LVO in a larger basilar artery.
Study limitations
The purpose of the study is to generate guidelines based on prior experience that will inform current practice and direct future studies. It is acknowledged that the guidelines derived here are not absolute and remain to be verified with an additional dataset from future patients at our institution or data from other institutions.
Limitations of the study include the retrospective design and the small numbers of the cohorts. Endovascular techniques have evolved dramatically during the period of the study and the EVT cohort included here received a variety of interventions ranging from intra-arterial urokinase to modern stentriever thrombectomy. It is expected that with modern techniques, the outcomes will improve.
Acknowledgment
We acknowledge Dr Hang Lee from the MGH Biostatistics center for assistance with data analysis.
Declaration of conflicting interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
References
- 1.Lindsberg PJ, Mattle HP. Therapy of basilar artery occlusion: A systematic analysis comparing intra-arterial and intravenous thrombolysis. Stroke 2006; 37: 922–928. [DOI] [PubMed] [Google Scholar]
- 2.Schonewille WJ, Wijman CA, Michel P, et al. Treatment and outcomes of acute basilar artery occlusion in the Basilar Artery International Cooperation Study (BASICS): A prospective registry study. Lancet Neurol 2009; 8: 724–730. [DOI] [PubMed] [Google Scholar]
- 3.Berkhemer OA, Fransen PS, Beumer D, et al. A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med 2015; 372: 11–20. [DOI] [PubMed] [Google Scholar]
- 4.Jovin TG, Chamorro A, Cobo E, et al. Thrombectomy within 8 hours after symptom onset in ischemic stroke. N Engl J Med 2015; 372: 2296–2306. [DOI] [PubMed] [Google Scholar]
- 5.Saver JL, Goyal M, Bonafe A, et al. Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. N Engl J Med 2015; 372: 2285–2295. [DOI] [PubMed] [Google Scholar]
- 6.Campbell BC, Mitchell PJ, Kleinig TJ, et al. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med 2015; 372: 1009–1018. [DOI] [PubMed] [Google Scholar]
- 7.Goyal M, Demchuk AM, Menon BK, et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med 2015; 372: 1019–1030. [DOI] [PubMed] [Google Scholar]
- 8.Muir KW, Ford GA, Messow CM, et al. Endovascular therapy for acute ischaemic stroke: The Pragmatic Ischaemic Stroke Thrombectomy Evaluation (PISTE) randomised, controlled trial. J Neurol Neurosurg Psychiatry 2017; 88: 38–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bracard S, Ducrocq X, Mas JL, et al. Mechanical thrombectomy after intravenous alteplase versus alteplase alone after stroke (THRACE): A randomised controlled trial. Lancet Neurol 2016; 15: 1138–1147. [DOI] [PubMed] [Google Scholar]
- 10.Yoon W, Kim SK, Heo TW, et al. Predictors of good outcome after stent-retriever thrombectomy in acute basilar artery occlusion. Stroke 2015; 46: 2972–2975. [DOI] [PubMed] [Google Scholar]
- 11.van Houwelingen RC, Luijckx GJ, Mazuri A, et al. Safety and outcome of intra-arterial treatment for basilar artery occlusion. JAMA Neurol 2016; 73: 1225–1230. [DOI] [PubMed] [Google Scholar]
- 12.Singer OC, Berkefeld J, Nolte CH, et al. Mechanical recanalization in basilar artery occlusion: The ENDOSTROKE study. Ann Neurol 2015; 77: 415–424. [DOI] [PubMed] [Google Scholar]
- 13.Higashida RT, Furlan AJ, Roberts H, et al. Trial design and reporting standards for intra-arterial cerebral thrombolysis for acute ischemic stroke. Stroke 2003; 34: e109–e137. [DOI] [PubMed] [Google Scholar]
- 14.Sims JR, Gharai LR, Schaefer PW, et al. ABC/2 for rapid clinical estimate of infarct, perfusion, and mismatch volumes. Neurology 2009; 72: 2104–2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Puetz V, Sylaja PN, Coutts SB, et al. Extent of hypoattenuation on CT angiography source images predicts functional outcome in patients with basilar artery occlusion. Stroke 2008; 39: 2485–2490. [DOI] [PubMed] [Google Scholar]
- 16.R Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing, 2013.
- 17.Gilberti N, Gamba M, Premi E, et al. Endovascular mechanical thrombectomy in basilar artery occlusion: Variables affecting recanalization and outcome. J Neurol 2016; 263: 707–713. [DOI] [PubMed] [Google Scholar]
- 18.Andersson T, Kuntze Söderqvist Å, Söderman M, et al. Mechanical thrombectomy as the primary treatment for acute basilar artery occlusion: Experience from 5 years of practice. J Neurointerv Surg 2013; 5: 221–225. [DOI] [PubMed] [Google Scholar]
- 19.Strbian D, Sairanen T, Silvennoinen H, et al. Thrombolysis of basilar artery occlusion: Impact of baseline ischemia and time. Ann Neurol 2013; 73: 688–694. [DOI] [PubMed] [Google Scholar]
- 20.Nagel S, Herweh C, Köhrmann M, et al. MRI in patients with acute basilar artery occlusion—DWI lesion scoring is an independent predictor of outcome. Int J Stroke 2012; 7: 282–288. [DOI] [PubMed] [Google Scholar]
- 21.Karameshev A, Arnold M, Schroth G, et al. Diffusion-weighted MRI helps predict outcome in basilar artery occlusion patients treated with intra-arterial thrombolysis. Cerebrovasc Dis 2011; 32: 393–400. [DOI] [PubMed] [Google Scholar]
- 22.Brandt T, von Kummer R, Müller-Küppers M, et al. Thrombolytic therapy of acute basilar artery occlusion. Variables affecting recanalization and outcome. Stroke 1996; 27: 875–881. [DOI] [PubMed] [Google Scholar]
- 23.Cross DT, 3rd, Moran CJ, Akins PT, et al. Relationship between clot location and outcome after basilar artery thrombolysis. AJNR Am J Neuroradiol 1997; 18: 1221–1228. [PMC free article] [PubMed] [Google Scholar]
- 24.Nagel S, Schellinger PD, Hartmann M, et al. Therapy of acute basilar artery occlusion: Intraarterial thrombolysis alone vs bridging therapy. Stroke 2009; 40: 140–146. [DOI] [PubMed] [Google Scholar]
- 25.Sairanen T, Strbian D, Soinne L, et al. Intravenous thrombolysis of basilar artery occlusion: Predictors of recanalization and outcome. Stroke 2011; 42: 2175–2179. [DOI] [PubMed] [Google Scholar]
- 26.Yoo AJ, Hakimelahi R, Rost NS, et al. Diffusion weighted imaging reversibility in the brainstem following successful recanalization of acute basilar artery occlusion. J Neurointerv Surg 2010; 2: 195–197. [DOI] [PubMed] [Google Scholar]
- 27.Haussen DC, Oliveira RA, Patel V, et al. Functional independence following endovascular treatment for basilar artery occlusion despite extensive bilateral pontine infarcts on diffusion-weighted imaging: Refuting a self-fulfilling prophecy. Interv Neurol 2016; 5: 179–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim YW, Hong JM, Park DG, et al. Effect of intracranial atherosclerotic disease on endovascular treatment for patients with acute vertebrobasilar occlusion. AJNR Am J Neuroradiol 2016; 37: 2072–2078. [DOI] [PMC free article] [PubMed] [Google Scholar]