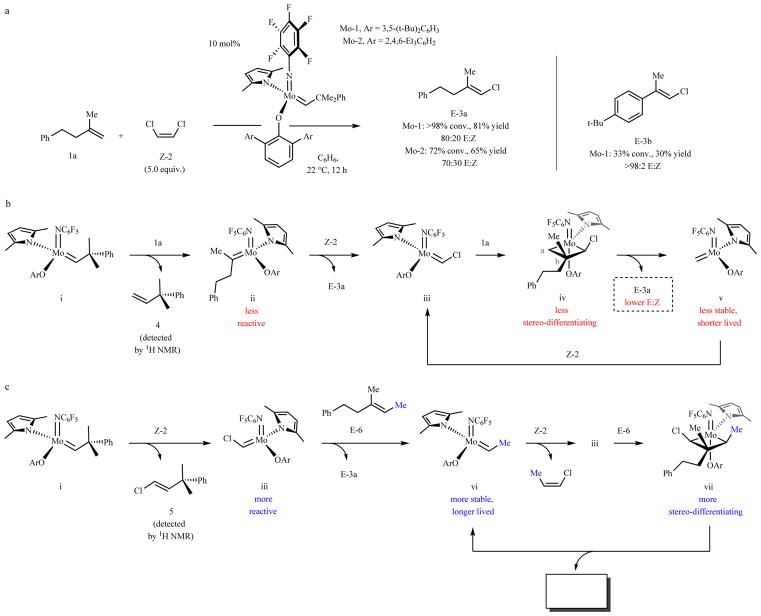
Fig. 1. The challenge of developing stereoselective trisubstituted alkene cross-metathesis.
a, Reaction between 1,1-disubstituted alkene 1a and Z-2 required 10 mol% loading for ≥72% conversion in 12 hours, affording E-3a in ≤80:20 E:Z ratio. Formation of E-3b was sluggish but more stereoselective due to substrate control. b, Inefficiency and low stereoselectivity is probably low stability of methylidene v and minimal size difference between the substituents in 1a. c, With a trisubstituted alkene (E-6), catalysis is initiated by reaction with Z-2 to generate iii, which is more robust than a methylidene complex. Moreover, the intermediacy of metallacyclobutane vii (vs. iv), should lead to superior stereoselectivity. Conv. and isomeric ratios determined by analysis of 1H NMR spectra of unpurified mixtures; yields are for isolated and purified products. See the Supplementary Information for details.