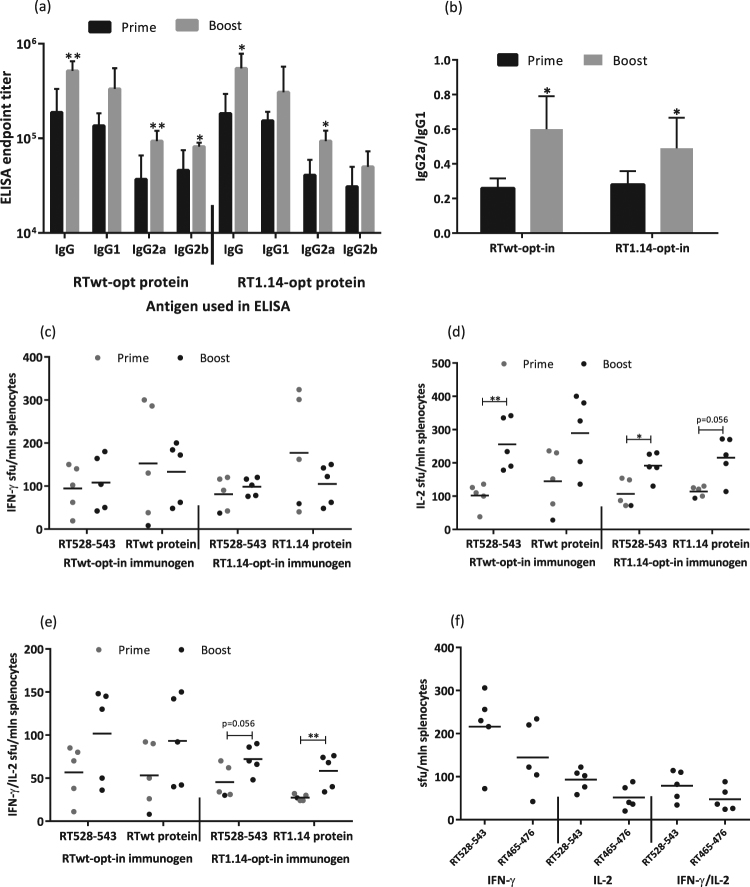
Figure 3.
RT-specific antibody and cellular responses after repeated immunization with expression-optimized RT genes. BALB/c mice (n = 5 or 6) were immunized with two intradermal injections (29G needle) containing 40 µg of RTwt-opt-in or RT1.14-opt-in encoding plasmids per mouse, with subsequent electroporation by Dermavax (standard protocol). Immunizations were repeated 4 weeks later with 20 µg of RTwt-opt-in or RT1.14-opt-in encoding plasmids ((a–e) prime-boost regimen, “Boost” on the graph) or 5 days later with 20 µg of RTwt-opt-in encoding plasmid ((f) double prime). Two independent immunizations were performed. At 21 days after the second immunization, mice were sacrificed and sera and splenocytes were isolated and subjected to immune tests. Endpoint average titers of anti-RT total IgG and IgG subtypes (the error bars represent the SD) were detected using ELISA against recombinant RTwt and RT1.14 proteins with cut-offs set against serum reactivity of control mice immunized with vector pVax1 (a), and the IgG2a/IgG1 ratio was calculated for antibody reactivity against RT variants matching RT used as the DNA immunogen (b). Splenocytes were stimulated in vitro with RT-derived peptides representing epitopes of RT aa 528–543 (c–f) and aa 465–476 ((f) Table 2) or recombinant RTwt and RT1.14 proteins ((c–e) matching the immunogen) in IFN-γ/IL-2 Fluorospot tests (c–f). The average number of cells was registered as signal-forming units (sfu) per million splenocytes secreting IFN-γ (c,f) IL-2 (d,f) and IFN-γ/IL-2 (e,f) and the error bars represent the SD. IFN-γ/IL-2 production by splenocytes of mice receiving prime-boost immunization compared to single immunization ((c–e) single immunization from Fig. 2) or double prime immunization with the RTwt-opt-in gene (f). All assays were performed in duplicate. *p < 0.05; **p < 0.01; p-values on the graph in the interval 0.05 to 0.1 indicate tendencies. Statistical comparisons were performed using Kruskal–Wallis and Mann–Whitney tests.