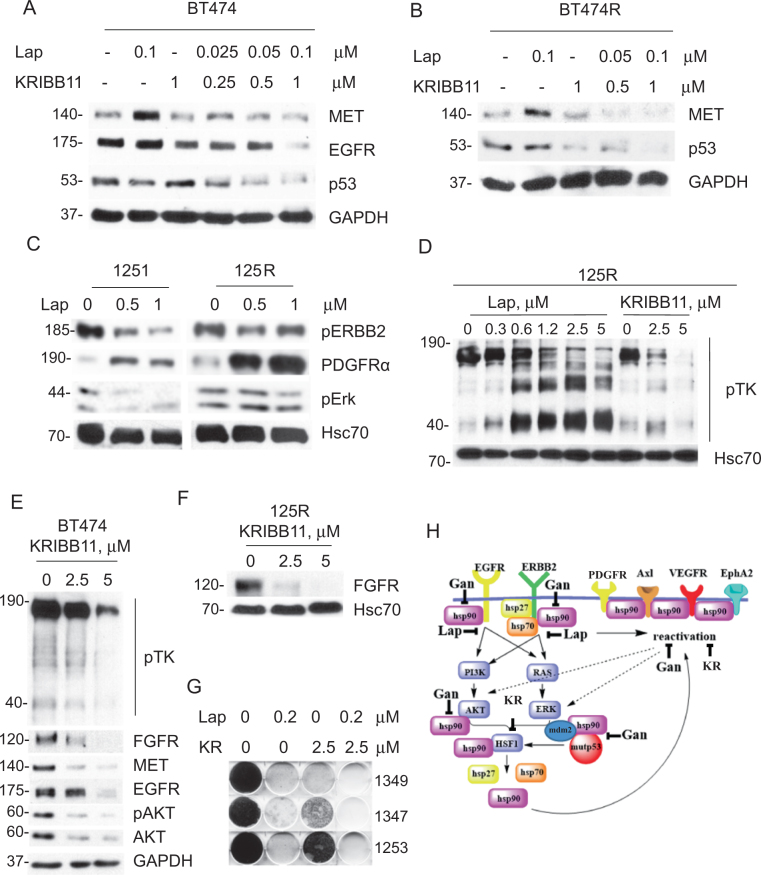
Fig. 6. HSF1 inhibition bypasses lapatinib-induced adaptive signaling and prevents the onset of lapatinib resistance.
a, b While lapatinib (0.1 µM, 48 h) upregulates MET in both lapatinib-sensitive BT474 (a) and lapatinib-resistant BT474R (b) cells, HSF1 inhibitor KRIBB11 reverses this effect. Moreover, KRIBB11 synergizes with lapatinib in degradation of EGFR and mutp53 (a) and restores responsiveness of mutp53 to lapatinib (b). Western blot analyses, GAPDH is a loading control. c–f Lapatinib (at indicated concentrations, 48 h) induces PDGFRα in both lapatinib-sensitive 1251 and lapatinib-resistant 125R murine cells (c) and induces global kinome activation (measured by phospho-Tyr antibody, pTK) in lapatinib-resistant 125R cells (d), KRIBB11 inhibits the global pTK activity and individual kinases in 125R cells (d, e), and BT474 cells (f). Western blot analysis, Hsc70 and GAPDH are loading controls. g KRIBB11 cooperates with lapatinib (at indicated concentrations, for 4 weeks) in suppressing emergence of lapatinib-resistant colonies. Colony formation assay. Representative images out of two technical replicas. h The proposed model. ERBB2 signaling mediates HSF1 activation4,16, which is potentiated by mutp53 via a feed-forward loop5,15, thereby upregulating Hsp90 clients including compensatory RTKs and mutp53 itself. Inhibition of ERBB2 by lapatinib leads to inhibition of HSF1 transcriptional activity and therefore decreased Hsp90 and release of Mdm2 from its inhibitory complex with Hsp904,5 and subsequent degradation of mutp53 and Mdm2. KRIBB11 simultaneously inhibits diverse adaptive RTKs, as well as destabilizes potent oncogenic drivers—ERBB2, EGFR, and mutp53