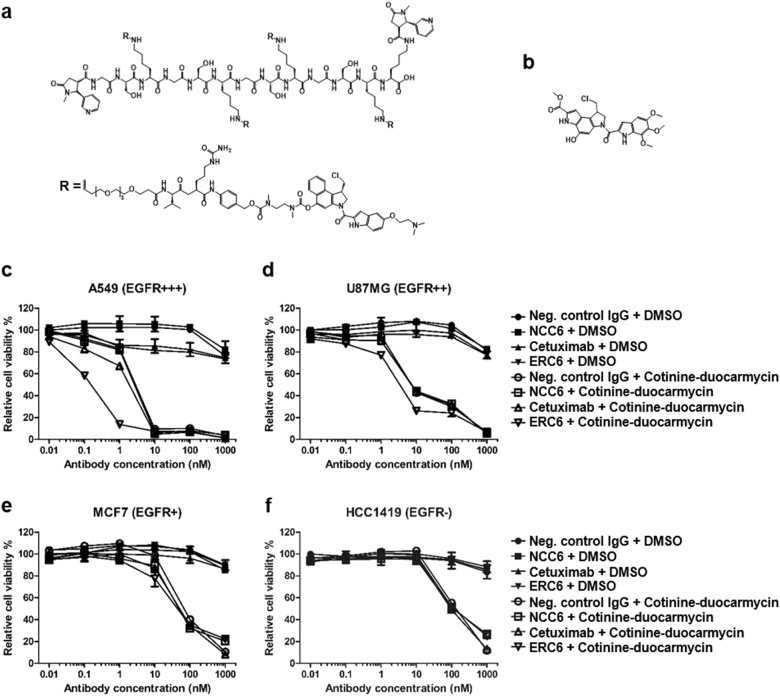
Fig. 5. The anti-proliferative effects of ERC6-complexed cotinine-duocarmycin on an EGFR-positive lung adenocarcinoma cell line.
a The chemical structure of bivalent cotinine-conjugated peptide crosslinked with four duocarmycins (cotinine-duocarmycin). R represents valine-citrullin p-aminobenzyloxycarbonyl (PAB)-linked dimethyl aminoethyl duocarmycin. b The chemical structure of free duocarmycin. c The A549, d U87MG, e MCF7, and f HCC1419 cells were treated with negative control IgG and DMSO (●); negative control IgG × cotinine bispecific antibody (NCC6) and DMSO (■); cetuximab and DMSO (▲); ERC6 and DMSO (▼); negative control IgG and cotinine-duocarmycin (○); NCC6 and cotinine-duocarmycin (□); cetuximab and cotinine-duocarmycin (△); or ERC6 and cotinine-duocarmycin (▽). DMSO was used as a vehicle control for cotinine-duocarmycin. After the cells were incubated for 72 h with the agents, the relative cell viability was determined by measuring the cellular ATP content using the Cell Titer-Glo reagent. The results are shown as the mean ± SD acquired from experiments conducted in triplicate