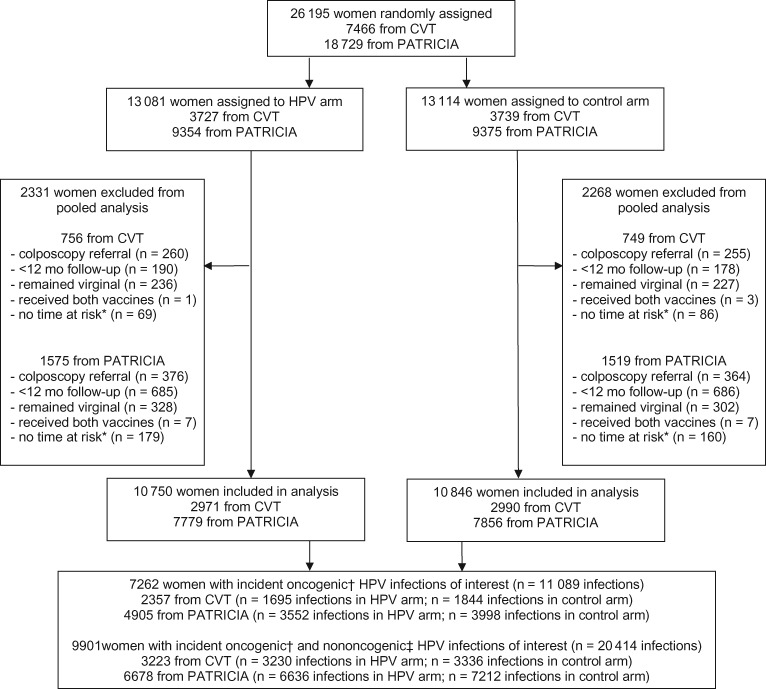
Figure 1.
Trial profile for Costa Rica Vaccine Trial (CVT) and PATRICIA trial pooled analysis. *Women may have contributed no time at risk because of prevalent infection with a vaccine-protected type that did not clear before their final study visit (excluded from analysis). †Oncogenic infections of interest include HPV types 35, 39, 51, 52, 56, 58, 59, and 68/73. ‡Nononcogenic HPV types of interest include HPV types 6, 11, 34, 40, 42, 43, 44, 53, 54, 66, 70, and 74. HPV = human papillomavirus.