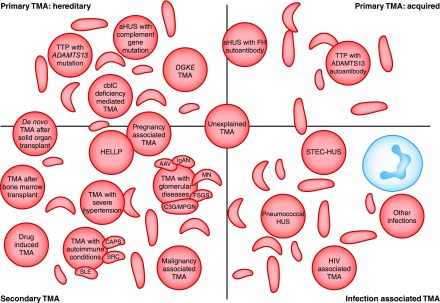
Figure 1.
Thrombotic microangiopathies are classified into: Inherited or acquired primary; secondary; or infection associated TMAs. Current classifications define primary TMAs as hereditary (mutations in ADAMTS13, MMACHC (cb1c deficiency), or genes encoding complement proteins) or acquired (autoantibodies to ADAMTS13, or autoantibodies to complement FH, which is associated with homozygous CFHR3/1 deletion). TMA is associated with various infections: in STEC-HUS and pneumococcal HUS, distinct mechanisms result in TMA; in other infections, the processes are not defined and in some cases the infection may trigger manifestation of a primary TMA. Secondary TMAs occur in a spectrum of conditions, and in many cases the pathogenic mechanisms are multifactorial or unknown. The classification presented here is not unequivocal: in some secondary TMAs, for example pregnancy-associated TMA or de novo TMA after transplantation, a significant proportion of individuals will have a genetic predisposition to a primary TMA. AAV, ANCA-associated vasculitis; ADAMTS13, a disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13; aHUS, atypical hemolytic uremic syndrome; C3G, C3 glomerulopathy; CAPS, catastrophic antiphospholipid syndrome; cblC, cobalamin C type; DGKE, gene encoding diacylglycerol kinase ε; FH, factor H; HELLP, syndrome of hemolysis, elevated liver enzymes, and low platelets; HUS, hemolytic uremic syndrome; IgAN, IgA nephropathy; MN, membranous nephropathy; MPGN, membranoproliferative GN; SRC, scleroderma renal crisis; STEC, shiga toxin–producing Escherichia coli; TMA, thrombotic microangiopathy; TTP, thrombotic thrombocytopenic purpura.