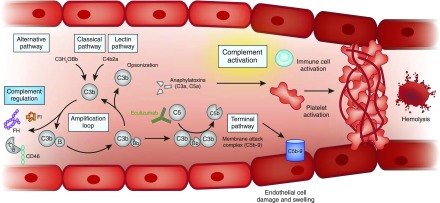
Figure 4.
Unfettered complement activation ultimately results in thrombus formation, platelet consumption, vascular occlusion and mechanical hemolysis. Complement is activated by the alternative, classic, and lectin pathways. The alternative pathway of complement is a positive amplification loop. C3b interacts with factor B, which is then cleaved by factor D to form the C3 convertase C3bBb. Unchecked, this leads to activation of the terminal complement pathway with generation of the effector molecules, the anaphylatoxin C5a and the membrane attack complex (C5b-9). To protect host cells from bystander damage, the alternative pathway is downregulated by complement regulators including factor H (FH), factor I (FI), and CD46. In complement-mediated aHUS, activating mutations in C3 and CFB and loss-of-function mutations in CFH, CFI, and CD46, in addition to autoantibodies to FH, result in overactivation of the alternative pathway. This leads to immune cell and platelet activation and endothelial cell damage and swelling, with consequent thrombus formation, platelet consumption, vascular occlusion, and mechanical hemolysis. There is evidence that complement is activated in other TMAs, but whether this is a causative, disease modifier, or bystander phenomenon has not yet been elucidated. Eculizumab is a humanized mAb that binds to C5 and prevents activation of the terminal pathway; it thereby inhibits the generation of the effector molecules that cause TMA in individuals in whom a primary defect in complement underlies the TMA pathogenesis. Its role in the treatment of other TMAs is undefined. aHUS, atypical hemolytic uremic syndrome; TMA, thrombotic microangiopathy.