Figure 2.
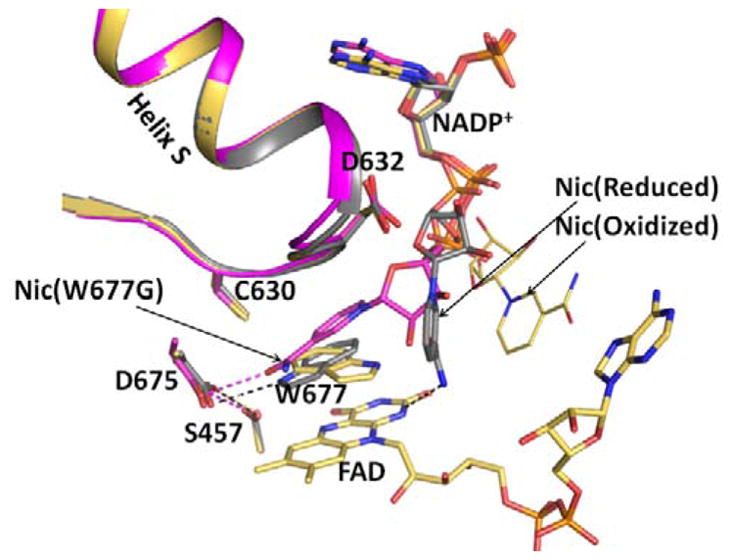
Structures of the oxidized triple mutant (Ser457Ala/Cys630Ala/Asp675Asn) CYPOR, (1JA1) (gold), dithionite-reduced CYPOR (grey), and oxidized Trp677Gly mutant (1J9Z, magenta) were superimposed. They reveal three conformations of the ribityl nicotinamide moiety of NADP+. In the oxidized wild type structure, the indole ring of Trp677 stacks onto the FAD ring and the ribityl nicotinamide (Nic-ox) is flexible (estimated occupancy, ~<50%) with no defined interactions with FAD and Trp677. In the reduced structure, the indole ring also stacks onto the flavin with the long axes of both rings being parallel. There is an H-bond between the Nε1 of Trp677 and Asp675 side chain. In addition, the carboxamide of the ribityl nicotinamide ring (Nic-reduced) forms hydrogen bonds with the negative N1 of the flavin and the Thr535 side chain hydroxyl. The face of the nicotinamide ring interacts with the edge of the Trp677 indole ring. In the Trp677Gly structure, the nicotinamide ring (Nic-W677G) stacks against the re-face of the isoalloxazine ring and is poised for hydride transfer. The three different ribityl-nicotinamide ring conformations may represent the nicotinamide binding pathway into the active site for hydride transfer, after securely anchoring the 2′, 5′-ADP half of NADP+. For clarity, only FAD of the oxidized structure is shown.
