Figure 4.
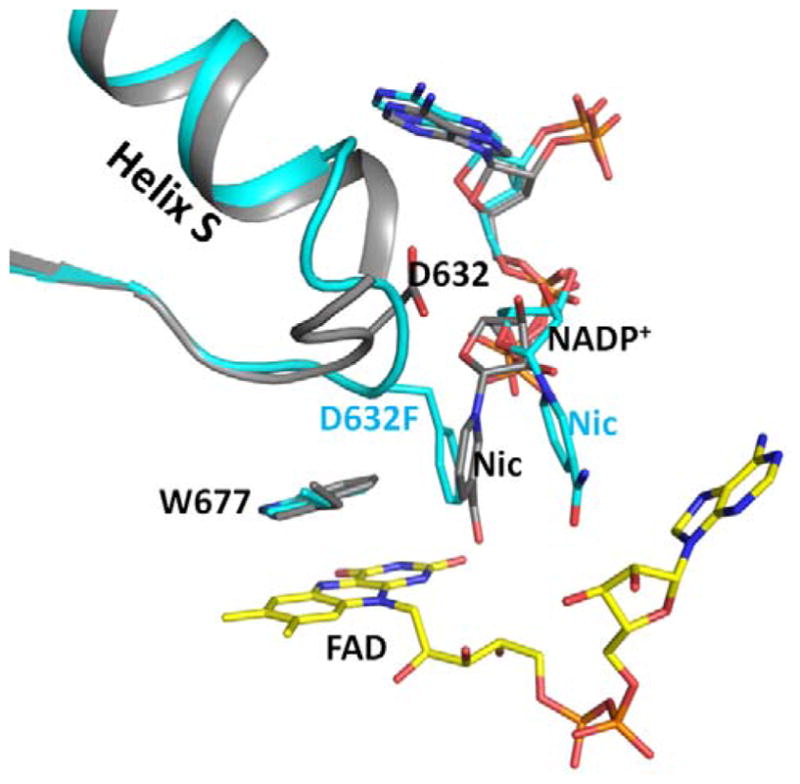
Superimposed structures of the reduced wild type (grey) and Asp632Phe mutant (cyan). The Asp632 loop conformations are different. The loop adopts the tight, retracted conformation in the reduced wild type structure, and a relaxed extended conformation in the Asp632Phe mutant structure. In the Asp632Phe structure, the side chain of Phe632 occupies the position of the nicotinamide ring of the reduced structure, and is almost perpendicular to the Trp677 indole ring in an edge to face conformation. The nicotinamide ring is essentially parallel to the phenyl ring in the Asp632Phe structure.
