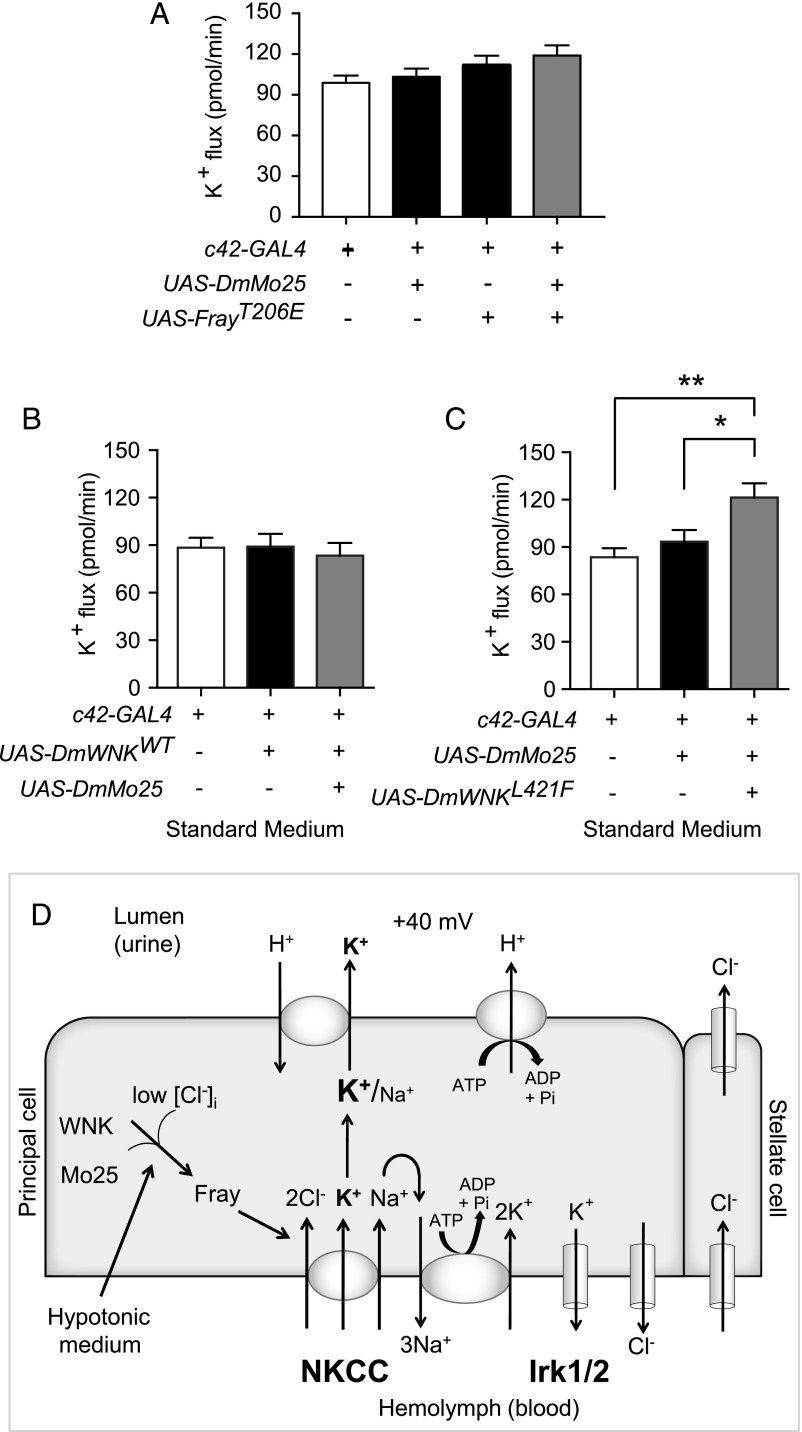
Figure 6.
Relief of chloride inhibition of With No Lysine kinase (WNK) and concomitant mouse protein 25 (Mo25) overexpression is sufficient to increase transepithelial ion flux in standard bathing medium. (A) Transepithelial potassium flux was measured using the Ramsay assay in standard bathing medium (isotonic conditions, approximately 30 mM intracellular chloride). Compared with control tubules, tubules in which FrayT206E was expressed during adulthood in principal cells with or without concomitant overexpression of D. melanogaster mouse protein 25 (DmMo25) had no difference in transepithelial potassium flux. Genotypes were control: w; tub-GAL80ts20/+; c42-GAL4/+ and experimental: w; tub-GAL80ts20 UAS-DmMo25/+; c42-GAL4/+, w; tub-GAL80ts20/UAS-FrayT206E; c42-GAL4/+, and w; tub-GAL80ts20 UAS-DmMo25/UAS-FrayT206E; c42-GAL4/+. Flies were reared at 18°C throughout development and shifted to 28°C for 2 days before testing to allow induction of the FrayT206E and DmMo25 transgenes; n=40–41 tubules per genotype. P=0.13, one-way ANOVA. (B) Compared with control tubules, tubules in which the wild type (WT) D. melanogaster With No Lysine kinase (DmWNK) was expressed during adulthood in principal cells with or without concomitant overexpression of DmMo25 had no difference in potassium flux. Genotypes were control: w; tub-GAL80ts20/+; c42-GAL4/+ and experimental: w; tub-GAL80ts20 UAS-DmWNKWT/+; c42-GAL4/+ and w; tub-GAL80ts20 UAS-DmWNKWT/UAS-DmMo25; c42-GAL4/+. Flies were reared at 18°C throughout development and shifted to 28°C for 2 days before testing to allow induction of the DmWNKWT and DmMo25 transgenes; n=24–29 tubules per genotype. P=0.16, one-way ANOVA. (C) Compared with control tubules, tubules overexpressing DmMo25 during adulthood had no difference in potassium flux, whereas tubules expressing both DmMo25 and chloride-insensitive DmWNKL421F had increased potassium flux. Genotypes were control: w; tub-GAL80ts20/+; c42-GAL4/+ and experimental: w; tub-GAL80ts20 UAS-DmMo25/+; c42-GAL4/+ and w; tub-GAL80ts20 UAS-DmMo25/UAS-DmWNKL421F; c42-GAL4/+. Flies were reared at 18°C throughout development and shifted to 28°C for 2 days before testing to allow induction of the DmWNKL421F and DmMo25 transgenes; n=24–25 tubules per genotype. P=0.002, one-way ANOVA. Adjusted P values (Tukey multiple comparisons test) were 0.62 for w; tub-GAL80ts20/+; c42-GAL4/+ versus w; tub-GAL80ts20 UAS-DmMo25/+; c42-GAL4/+, 0.03 for w; tub-GAL80ts20 UAS-DmMo25/+; c42-GAL4/+ versus w; tub-GAL80ts20 UAS-DmMo25/UAS-DmWNKL421F; c42-GAL4/+ and 0.002 for w; tub-GAL80ts20/+; c42-GAL4/+ versus w; tub-GAL80ts20 UAS-DmMo25/UAS-DmWNKL421F; c42-GAL4/+. *P=0.03; **P=0.002. (D) Model. The principal cell apical vacuolar H+-ATPase drives fluid secretion65 by extruding protons to generate a lumen-positive transepithelial potential difference.16 This is thought to drive exchange of protons for cations, primarily potassium in the Drosophila renal tubule. Transepithelial chloride secretion through the stellate cells is also driven by the lumen-positive charge.16,17 Both the sodium-potassium 2 chloride cotransporter (NKCC), encoded by Ncc69, and inwardly rectifying potassium channels, Irk1 and Irk2, are required for transepithelial potassium flux.19,21 Sodium entering the epithelial cells through NKCC is recycled through the Na+/K+-ATPase.19 There is also a basolateral chloride conductance with molecular identity that is unknown.48 WNK and Fray (the Drosophila Ste20-related proline/alanine-rich kinase [SPAK]/oxidative stress response 1 [OSR1] homolog) are positive regulators of potassium flux through NKCC, and hypotonicity stimulates transepithelial potassium flux in a WNK/Fray/NKCC-dependent manner.18 Here, we show that intracellular chloride concentrations in renal tubule epithelial cells fall in hypotonic conditions, with a concomitant increase in tubule WNK activity. Mo25 is also required for maximal ion transport. A mutation that abolishes chloride inhibition of WNK together with Mo25 overexpression is sufficient to stimulate transepithelial ion flux. These results implicate both chloride and Mo25 as important regulators of WNK signaling in a transporting renal epithelium.