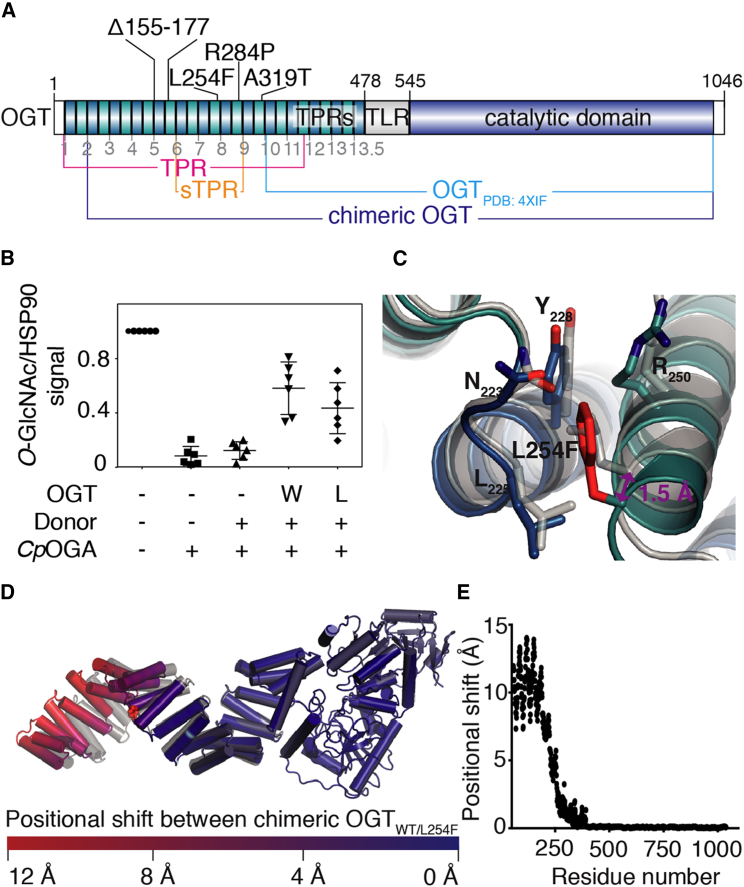
Figure 1.
In Vitro Characterization of OGTL254F
(A) Schematic representation of OGT highlighting the intellectual disability-associated mutations and all the constructs used in this study.
(B) Scatterplot showing OGT activity against deglycosylated HEK-293 cell lysate, with the data averaged from six replicates and the error bars showing SD. See also Figure S1B.
(C) Superposition of the TPRWT/L254F crystal structures at the site of mutation. The gray and colored cartoons are that of TPRWT (PDB: 1W3B; Jínek et al., 2004) and TPRL254F (PDB: 6EOU) structures, respectively.
(D) Overlay of the chimeric OGTWT/L254F structures. The wild-type structure is colored gray, while the mutant structure is colored to reflect the positional shift of each Cα atom between the two structures.
(E) Graph showing the positional shift between equivalent Cα atoms between chimeric OGTWT (PDB: 4XIF [Pathak et al., 2015] and PDB: 1W3B [Jínek et al., 2004]) and OGTL254F (PDB: 4XIF [Pathak et al., 2015] and PDB: 6EOU) as a function of residue number.
L, L254F; OGT, O-GlcNAc transferase; sTPR, simplified TPR; TPR, tetratricopeptide repeat; W, wild-type.