Figure 3.
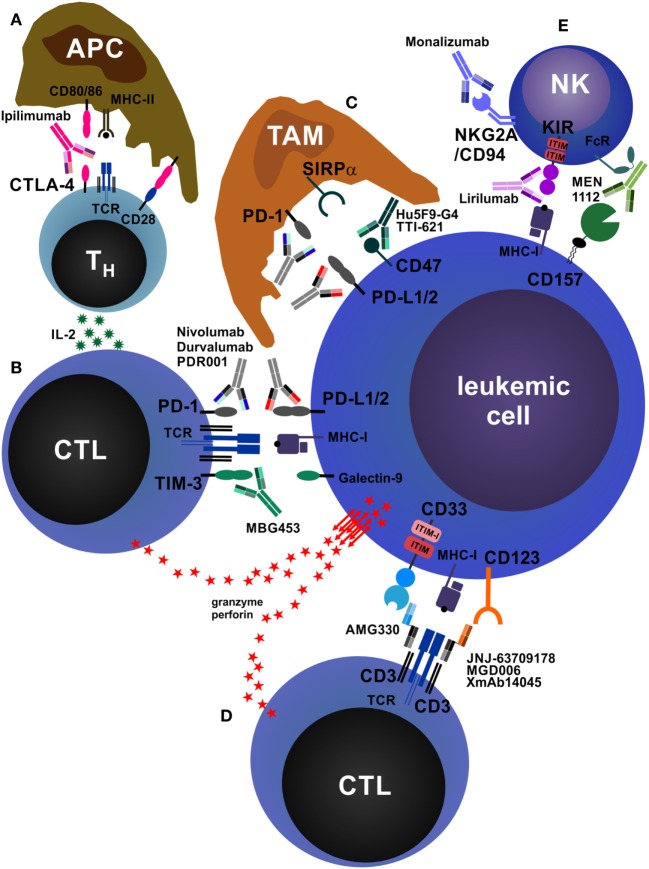
Antibodies that reinforce host immunity. (A) In secondary lymphoid organs, antigen-presenting cells (APCs) process and present peptides from tumor-derived (neo-)antigens to CD4+ helper T cells (TH cells) via major histocompatibility complex class II (MHC-II) molecules. The second signal required for T cell activation is CD28, which is stimulated by CD80/86 expressed on APCs. TH cells produce interleukin-2 (IL-2), increasing proliferation and survival of both TH cells and CD8+ cytotoxic T cells (CTLs). In addition, APCs may also directly activate tumor-specific CTLs via cross-presentation of tumor peptides on MHC-I (not depicted). After activation and clonal expansion, T cells upregulate the inhibitory receptor cytotoxic T lymphocyte antigen-4 (CTLA-4). The monoclonal antibodies (mAbs) ipilimumab and tremelimumab block inhibitory CTLA-4 signals on T cells and concomitantly redirect CD80/86 signals from APCs to CD28, enhancing T cell activation. (B) CTLs recognize acute myeloid leukemia (AML) cells via T cell receptor (TCR)–MHC-I interactions. AML cells express the inhibitory molecules PD-L1/2 and galectin-9, which drive programmed death protein-1 (PD-1)- and TIM-3-expressing CTLs into anergy/exhaustion. Anti-PD-1 and anti-PD-L1/2 mAbs (nivolumab, pembrolizumab, atezolizumab, durvalumab, PDR001, etc.) and anti-TIM-3 mAbs (MBG453) prevent the exhaustion of AML-specific CTLs and improve AML cell killing via perforin/granzyme. In addition, AML cells can themselves express TIM-3, which is stimulated by galectin-9 in an autocrine loop and promotes their self-renewal (not depicted) (210). In addition, PD-1 is also expressed on tumor-infiltrating CD4+FOXP3+ regulatory T cells (Treg cells), and PD-1 signaling on Treg cells enhances their proliferation (not depicted) (211). (C) Tumor-associated macrophages (TAMs) are a highly heterogeneous cell population in the tumor microenvironment that crucially influences tumor biology. TAMs can be broadly categorized into M1 (inflammatory) and M2 (pro-tumorigenic) macrophages. TAMs, especially M2 TAMs, express PD-1, which inhibits their phagocytic function. In addition, almost all tumors overexpress the “don’t eat me” molecule CD47, reinforcing the antiphagocytic state of TAMs by triggering signal regulatory protein α (SIRPα). Blocking PD-1, PD-L1/2, and CD47 by therapeutic mAbs enhances tumor cell phagocytosis. (D) Bispecific antibodies (BsAbs) are hybrid molecules with two different antigen specificities, of which one is targeted against a T cell surface molecule (most often CD3) and the other against an AML cell surface molecule, such as CD33 (AMG330), CD123 (JNJ-637079178, MGD006, XmAb14045), or C type lectin-like molecule-1/CLEC12A (MCLA-117, not depicted). BsAbs facilitate activation and killing by promoting adhesion of T cells to AML cells. (E) Natural killer (NK) cells play an important role in antitumor immunity. They can kill tumor cells directly and produce immunostimulatory cytokines. The activation of NK cells is normally tightly controlled by inhibitory receptors, such as killer cell immunoglobulin-like receptor (KIR) and NKG2A/CD94. Tumor cells lacking MHC-I and/or human leukocyte antigen-E, the ligands for NK cell inhibitory receptors, potently activate and are killed by NK cells. Blocking these inhibitory receptors by mAbs such as lirilumab (anti-KIR) or monalizumab (anti-NKG2A/CD94) mimics this effect. In addition, NK cells express Fc receptors (FcRs) and recognize antibody-opsonized tumor cells, leading to antibody-dependent cell-mediated cytotoxicity. As an example, CD157 is shown.