Abstract
A pancreatic collision tumor is a rare entity that can be challenging to diagnose. We present a very rare case of a pancreatic collision tumor composed of both a neuroendocrine tumor and a ductal adenocarcinoma. Preoperative diagnosis was clinically challenging because both the radiology and fine-needle biopsy were consistent with a typical neuroendocrine mass. However, gross examination of the mass postoperatively revealed neuroendocrine cells with rare foci of ductal adenocarcinoma without a transition zone. Awareness of this entity is important so that medical practitioners consider pursuing surgical management of pancreatic lesions that otherwise would be managed exclusively with surveillance.
Introduction
A collision tumor is a malignancy that is comprised of at least two types of cancers in the same anatomical site, with no mixed area within the transition zone.1,2 A collision tumor within the pancreas is an extremely rare entity and usually has a poor prognosis, even after radical resection.3
Case Report
A 51-year-old woman with hypertension and diabetes presented with a 7-month history of progressively worsening right upper quadrant abdominal pain. She denied history of acute or chronic pancreatitis, and denied any substance abuse. On exam, she had moderate tenderness to palpation in the right upper quadrant. Laboratory examination revealed normal serum alanine aminotransferase, alkaline phosphatase, total bilirubin, lipase, CA19-9, and immunoglobulin G subclass 4 levels.
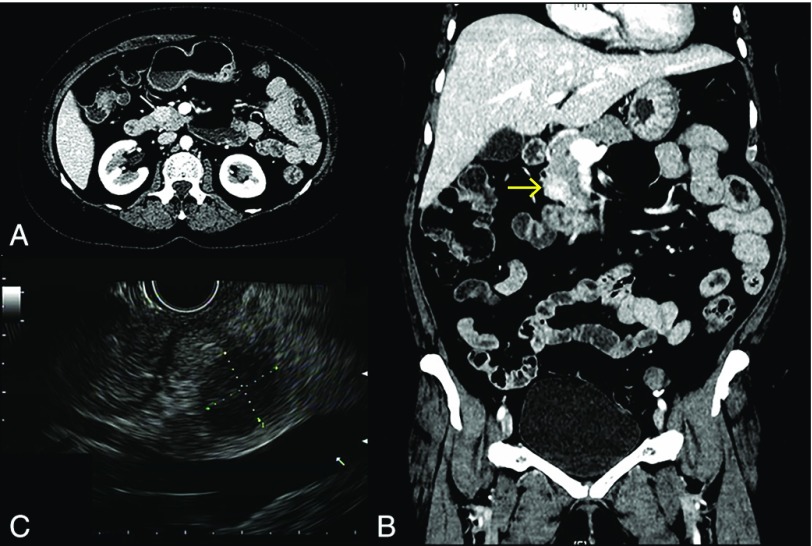
Contrast-enhanced computed tomography (CT) of the abdomen showed a well-circumscribed, heterogeneously enhancing lesion (1.7 × 1.5 cm) within the pancreatic head, with no dilation of the pancreatic duct or common bile duct (Figure 1). Endoscopic ultrasound revealed a well-demarcated, round, hypoechoic mass, measuring 1.8 × 1.55 cm in its largest dimension, as well as a non-dilated pancreatic duct and normal-appearing pancreatic body and tail (Figure 1). Fine-needle aspiration biopsy revealed uniform cells with round, regular nuclei and finely distributed chromatin without prominent nucleoli, arranged in single cells as well as in sheets and clusters, with rare groups of benign glandular epithelium (Figure 2). Immunohistochemistry was positive for CE-45, chromogranin, neuron-specific enolase, and synaptophysin, all suggestive of a diagnosis of a pancreatic neuroendocrine neoplasm.
Figure 1.
Radiology features of the pancreatic lesion. Contrast-enhanced computed tomography (A) axial and (B) coronal image demonstrating a heterogeneously enhanced hypervascular lesion (arrow) in the head of the pancreas. (C) Endoscopic ultrasound image demonstrating the mass within the head of the pancreas (1.8 × 1.55 cm).
Figure 2.
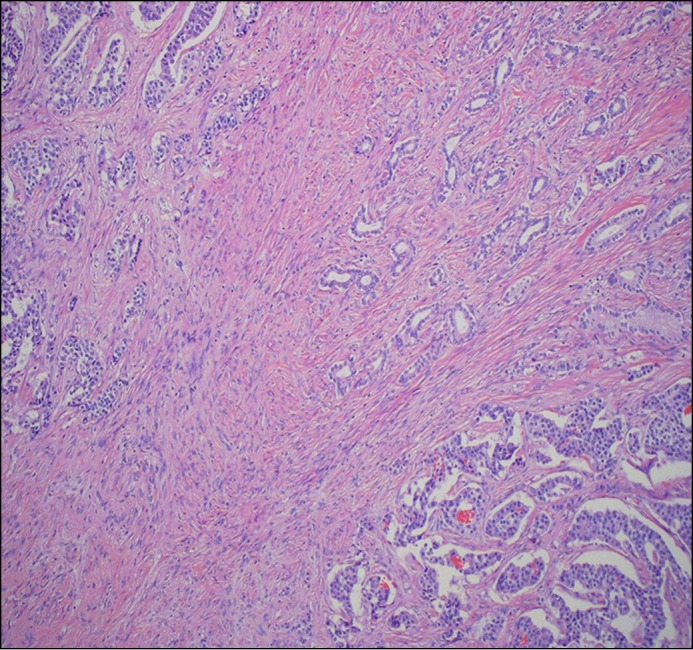
Low-power view of an area demonstrating components of neuroendocrine tumor (upper left and lower right) and pancreatic ductal adenocarcinoma (PDAC; upper right), without a transition zone.
Gross pathology of the specimen obtained with a Whipple procedure revealed nests of cells separated by thick fibrous bands. Microscopically, the majority of the tumor consisted of nests of cells that were positive for chromogranin and synaptophysin but negative for CA 19-9, consistent with a well-differentiated neuroendocrine tumor (NET) (Figure 3). However, located within the center of this lesion were small infiltrating glands with cellular atypia and surrounding desmoplastic stroma. These cells were positive for CA 19-9 and negative for chromogranin and synaptophysin, consistent with the diagnosis of a concurrent pancreatic ductal adenocarcinoma (PDAC) within the NET (Figure 4). Ki-67 staining also demonstrated staining in less than 2% of the lesion.
Figure 3.

Microscopic view of well-differentiated neuroendocrine tumor. (A) High-power view with tumor cells forming irregular nests and trabecular cords. (B) Immunohistochemical stain for chromogranin revealed positivity (brown) in the neuroendocrine tumor component but not in the PDAC component.
Figure 4.

Microscopic view of well-differentiated PDAC. (A) High-power view showing infiltration of small, irregular glands with nuclear atypia and surrounding fibrous tissues. (B) Immunohistochemical stain for CA 19-9 revealed positivity (brown) in the PDAC component but not in the neuroendocrine tumor component.
The patient had an uneventful recovery. Surveillance contrast-enhanced abdominal CT performed every 6 months revealed no signs of recurrence of cancer at 12 months after surgical resection.
Discussion
We report an exceedingly rare case of a collision pancreatic tumor consisting of NET and PDAC. According to the World Health Organization histological classification, collision tumors are composed of at least 2 different malignant components, separated by stroma without histological admixture.4 Collision tumors can occur in any organ of the body, and have been reported in the uterus, adrenal gland, esophagus, stomach, colon, breast, and ovary, but they most commonly occur in the stomach and esophagus.1,5-10 Pancreatic collision tumors are very rare, and the literature is therefore limited. There is only one similar case in the literature of a pancreatic collision tumor found in an elderly woman.11 In that case, the preoperative diagnosis was an intraductal papillary mucinous neoplasm based on radiological findings.11 Gross pathology revealed a PDAC in the entirety of the pancreas and a NET within the head of the pancreas.11 Indeed, the diagnosis of a collision tumor is usually a postoperative diagnosis based on the pathological examination of the surgical specimen, as there may be no specific symptoms or radiological features before surgery.3
Little is known about the mechanism of carcinogenesis of collision cancers; there are, however, several proposed theories in the literature. One hypothesis is that there may be a dysfunction of multiple tumor-suppressor genes that causes inadequate repair of genes, resulting in multiple types of malignancies.3,12,13 Other theories postulate that different tumors can originate from totipotent endodermal or intermediate cells, which are able to differentiate into both endocrine and ductular structures as demonstrated in experimental carcinogenesis of rodents.14 The concept of a field effect or field carcinogenesis, described in the field of head and neck cancers, proposes that within a field of normal tissue with genetic predisposition, an environmental carcinogen can create a cellular and molecular alterations, thereby predisposing the development of neoplasms within that territory.15,16 The concept of epigenetic alterations, somatic mutations induced by carcinogens, as well as heritable mutations in cancer susceptibility genes has become a well-accepted theory of carcinogenesis in any part of the body. The hypothesis of field carcinogenesis could be a possible explanation for synchronous and metachronous lesions in the pancreas and throughout the gastrointestinal tract.
From the small number of reported cases, collision tumors appear to have a poor prognosis.17 One case series reported a median survival of 10 months for pancreatic and peri-ampullary collision tumors even after radical resection, which was significantly lower than the 27 months in patients with pancreatic adenocarcinoma who received radical surgery.3 Surgical resection appears to be a rational method of treatment for collision tumors amenable to resection based on prior reported cases. However, a preoperative diagnosis can be nearly impossible to make, and collision tumors are typically diagnosed postoperatively.17 The current National Comp-rehensive Cancer Network guidelines recommend surveillance rather than surgical management for small (<1 cm), incidentally found pancreatic NET.18 It has been suggested in the literature that special attention should be paid to atypical radiologic features such as heterogeneity on CT imaging, presence of surrounding low-density areas, or abnormal accumulation in fluorodeoxyglucose-positron emission tomography.19 It remains to be determined whether these features efficiently predict collision cancer, and if surgical resection improves overall survival even if the diagnosis is confirmed.
We report a rare case of a pancreatic collision tumor consisting of a NET with a small focus of PDAC. The exact mechanism of tumorigenesis remains unclear, although there are several proposed hypotheses. When faced with a pancreatic mass with atypical radiologic features, the diagnosis of a collision tumor should be considered, and surgical resection may be a rational treatment method.
Disclosures
Author contributions: Y. Wang and S. Gandhi wrote and edited the manuscript. A. Ijeli wrote the manuscript. A. Basu, P. Kovarik, M. Sekosan, and M. Demetria edited the manuscript. Y. Wang is the article guarantor.
Financial disclosure: None to report.
Informed consent was obtained for this case report.
References
- 1.Kim SH, Kim YJ, Park BK, Cho JY, Kim BH, Byun JY. Collision tumors of the ovary associated with teratoma: Clues to the correct preoperative diagnosis. J Comput Assist Tomogr. 1999;23(6):929–33. [DOI] [PubMed] [Google Scholar]
- 2.Allen C, Stephens M, Williams J. Combined high grade sarcoma and serous ovarian neoplasm. J Clin Pathol. 1992;45(3):263–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Niu GM, Jin DY, Ji Y, Hou J, Wang DS, Lou WH. Survival analysis of pancreatic and periampullary collision cancers. J Dig Dis. 2010;11(4):231–36. [DOI] [PubMed] [Google Scholar]
- 4.Hamilton SR, Aaltonen LA. WHO classification of tumours. Pathology and genetics of tumours of the digestive system. Geneva: World Health Organization; 2000. [Google Scholar]
- 5.Gaertner EM, Farley JH, Taylor RR, Silver SA. Collision of uterine rhabdoid tumor and endometrioid adenocarcinoma: a case report and review of the literature. Int J Gynecol Pathol. 1999;18(4):396–401. [DOI] [PubMed] [Google Scholar]
- 6.Liu SW, Chen GH, Hsieh PP. Collision tumor of the stomach: A case report of mixed gastrointestinal stromal tumor and adenocarcinoma. J Clin Gastroenterol. 2002;35(4):332–34. [DOI] [PubMed] [Google Scholar]
- 7.Schwartz LH, Macari M, Huvos AG, Panicek DM. Collision tumors of the adrenal gland: Demonstration and characterization at MR imaging. Radiology. 1996;201(3):757–60. [DOI] [PubMed] [Google Scholar]
- 8.Spagnolo DV, Heenan PJ. Collision carcinoma at the esophagogastric junction: Report of two cases. Cancer. 1980;46(12):2702–8. [DOI] [PubMed] [Google Scholar]
- 9.Lyda MH, Fenoglio-Preiser CM. Adenoma-carcinoid tumors of the colon. Arch Pathol Lab Med. 1998;122(3):262.. [PubMed] [Google Scholar]
- 10.Susnik B, Jordi Rowe J, Redlich PN, Chitambar C, Chang CC, Kampalath B. A unique collision tumor in breast: invasive ductal carcinoma and mucosa-associated lymphoid tissue lymphoma. Arch Pathol Lab Med. 2004;128(1):99–101 . [DOI] [PubMed] [Google Scholar]
- 11.Serafini S, Da Dalt G, Pozza G, Blandamura S, Valmasoni M, Merigliano S, Sperti C. Collision of ductal adenocarcinoma and neuroendocrine tumor of the pancreas: A case report and review of the literature. World J Surg Oncol. 2017;15(1):93.[Mismatch] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hashimoto Y, Murakami Y, Uemura K, et al. Mixed ductal‐endocrine carcinoma derived from intraductal papillary mucinous neoplasm (IPMN) of the pancreas identified by human telomerase reverse transcriptase (hTERT) expression. J Surg Oncol. 2008;97(5):469–75. [DOI] [PubMed] [Google Scholar]
- 13.Chial H. Tumor suppressor (TS) genes and the two-hit hypothesis. Nature Educ. 2008;1(1):177. [Google Scholar]
- 14.Stukavec J, Jirasek T, Mandys V, et al. Poorly differentiated endocrine carcinoma and intraductal papillary-mucinous neoplasm of the pancreas: Description of an unusual case. Pathol Res Pract. 2007;203(12):879–84. [DOI] [PubMed] [Google Scholar]
- 15.Bernstein C, Bernstein H, Payne CM, Dvorak K, Garewal H. Field defects in progression to gastrointestinal tract cancers. Cancer Lett. 2008;260(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Slaughter DP, Southwick HW, Smejkal W “Field cancerization” in oral stratified squamous epithelium. Clinical implications of multicentric origin. Cancer. 1953;6(5):963–68. [DOI] [PubMed] [Google Scholar]
- 17.Kim HJ, Choi BG, Kim CY, Cho CK, Kim JW, Lee JH, Hur YH. Collision tumor of the ampulla of Vater: coexistence of neuroendocrine carcinoma and adenocarcinoma: report of a case. Korean J Hepatobiliary Pancreat Surg. 2013;17(4):186–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kulke MH, Shah MH, Benson AB, et al. Neuroendocrine tumors, version 1.2015. J Natl Compr Canc Netw. 2015;13(1):78–108. [DOI] [PubMed] [Google Scholar]
- 19.Araki K, Shimura T, Kobayashi T, et al. Mixed ductal-endocrine carcinoma of the pancreas occurring as a double cancer: Report of a case. Int Surg. 2011;96(2):153–58. [DOI] [PubMed] [Google Scholar]