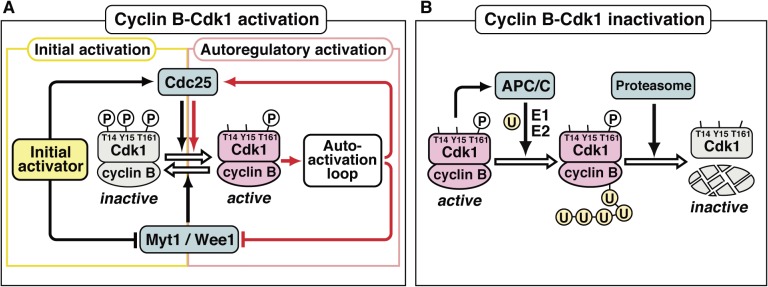
Figure 2.
Activation of the cyclin B-Cdk1 complex at entry into M-phase and its subsequent inactivation at exit from M-phase. Cdc2 was renamed as cyclin-dependent kinase 1 (Cdk1) in the designation of the Cdk family in 1991.78) (A) At the G2/M-phase border, Myt1/Wee1 (which phosphorylates cyclin B-associated Cdk1 on Thr14 and Tyr15 for inhibition) surpasses Cdc25 (which dephosphorylates cyclin B-associated Cdk1 on the sites phosphorylated by Myt1/Wee1 for activation), and accordingly, cyclin B-Cdk1 remains in an inactive form, in which all three sites on Cdk1 are phosphorylated (indicated by the circled P). At the transition into M-phase, the initial activator (which is different depending on the cell type) first tips the balance between Myt1/Wee1 and Cdc25 activities to trigger the initial activation of cyclin B-Cdk1. Subsequently, active cyclin B-Cdk1 directly phosphorylates Cdc25 and Myt1/Wee1 for activation and inhibition, respectively, and hence the Myt1/Wee1-Cdc25 balance is further reversed via an autoactivation loop, leading to the robust and full autoregulatory activation of cyclin B-Cdk1. Details on the autoactivation loop are shown in Fig. 9. (B) At exit from M-phase, active cyclin B-Cdk1 activates an E3 ligase, the anaphase-promoting complex/cyclosome (APC/C), leading to poly-ubiquitination of Cdk1-associated cyclin B. Subsequently, the proteasome recognizes the poly-ubiquitin chain, dissociates poly-ubiquitinated cyclin B from Cdk1, and then proteolyses cyclin B, resulting in inactivation of cyclin B-Cdk1. U, ubiquitin; E1, ubiquitin-activating enzyme; E2, ubiquitin-conjugating enzyme.