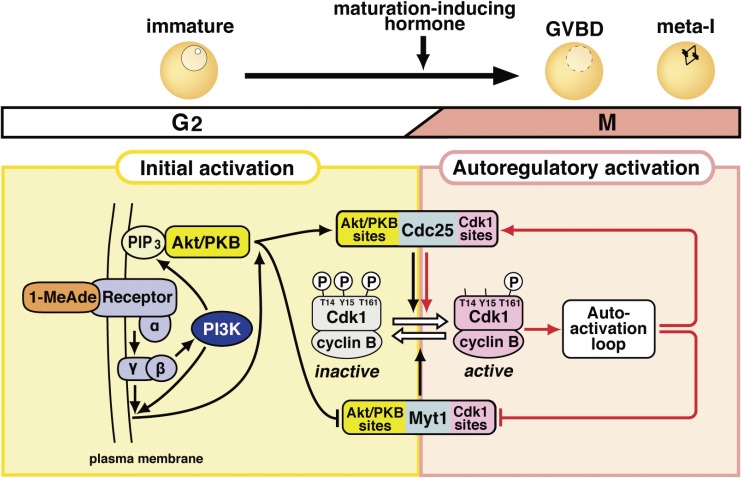
Figure 4.
Signaling pathway leading to the activation of cyclin B-Cdk1 at the meiotic G2/M-phase transition in starfish oocytes. This pathway consists of the initial activation of cyclin B-Cdk1 and its subsequent autoregulatory activation, as shown in Fig. 2A. The initial activation pathway may be characteristic of the starfish oocyte system, whereas the autoregulatory activation pathway is largely conserved. The putative 1-MeAde receptor on the oocyte surface couples with heterotrimeric G-protein, from which the Gβγ complex is released to cause the initial activation of cyclin B-Cdk1 via two parallel pathways. In one pathway, Gβγ activates phosphoinositide 3-kinase (PI3K) to produce phosphatidylinositol 3,4,5-triphosphate (PIP3), depending on which Akt/protein kinase B (PKB) is activated. Akt/PKB directly phosphorylates Myt1 and Cdc25 for downregulation and upregulation, respectively. In the other pathway, Gβγ along with PI3K contributes, via unknown molecule(s), to the phosphorylation of Cdc25 and Myt1 on residues phosphorylated by Akt/PKB (Akt/PKB sites). These initial phosphorylations on the Akt/PKB sites, which are accomplished by possible cooperation of these two pathways, tip and reverse the balance between Cdc25 and Myt1 activities, leading to activation of a small population of cyclin B-Cdk1. The autoactivation loop then starts the activation of a much larger population of cyclin B-Cdk1 (see Fig. 9 for details). In the autoregulatory activation, Cdc25 and Myt1 are directly phosphorylated largely by cyclin B-Cdk1 (Cdk1 sites).