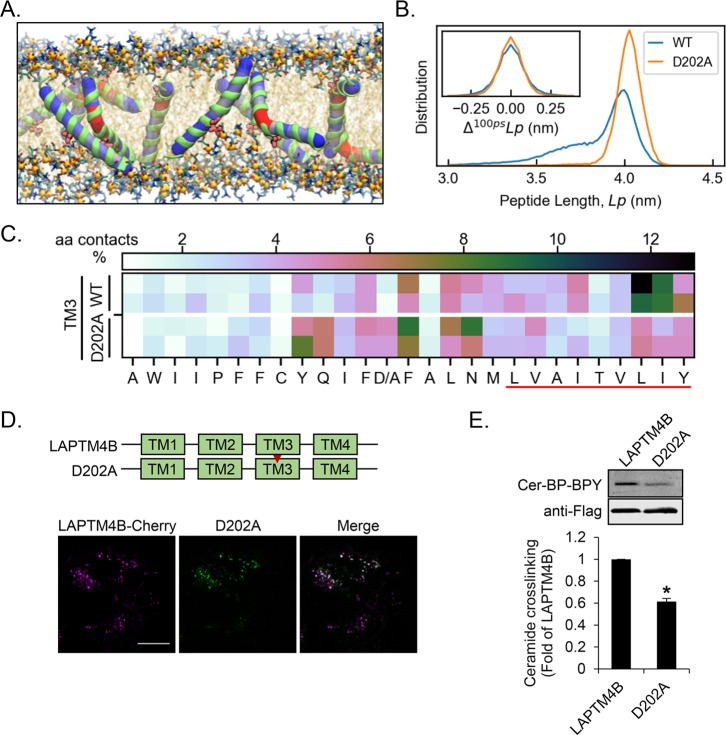
Figure 3.
Aspartate 202 in TM3 of LAPTM4B is central for the ceramide interaction. (A) LAPTM4B-derived TM3 can bend in the region surrounding the central aspartate shown in red (van der Waals representation). (B) The D202A mutation reduces the flexibility of TM3 (increased peptide length) and dynamics (change in peptide length per 100 ps interval) compared to the WT. (C) Ceramide interaction with WT-TM3 and the D202A mutant. The WT simulation data are the same as in Figure 2A and included for comparison. The red line highlights the sphingolipid-binding motif. (D) Schematic representation of LAPTM4B WT and the D202A point mutant. The cellular localization of LAPTM4B and the D202A mutant in A431 cells was visualized by immunofluorescence microscopy. Scale bar 20 μm. (E) Cross-linking of Cer-BP-BPY to LAPTM4B and D202A mutant was assessed by immunoprecipitation, in-gel fluorescence imaging of cross-linked Cer-BP-BPY, and immunoblotting of precipitated Flag-tagged LAPTM4B. Top panel, representative experiment; bottom panel, quantification of cross-linked Cer-BP-BPY for D202A compared to LAPTM4B (n = 3 experiments, mean ± SEM, p* < 0.05).