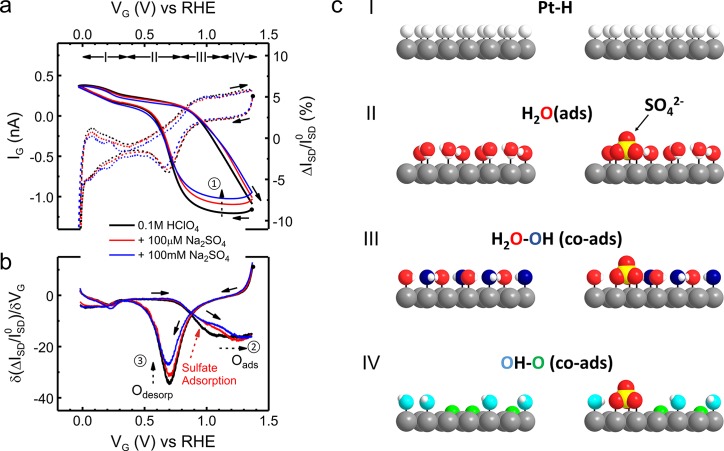
Figure 2.
In situ electrical transport spectroscopy (ETS) of sulfate adsorption on Pt surface. (a) IG–VG (on-chip CV, dashed curves) and normalized ISD–VG (ETS, solid curves) characteristics of a typical PtNW device in 0.1 M HClO4 (black) and with addition of varying concentrations of sulfate anions (red and blue). The CV results resemble the typical CV characteristic of a polycrystalline Pt surface, containing redox regions of under potential hydrogen adsorption (region I); the double layer (region II), reversible adsorption of OH (region III), and surface oxide formation (region IV). (b) Differentiated analysis of ETS (dETS) results showing spectral peak characteristics. Dashed arrows (1 in a and 2, 3 in b) indicate the change of ETS or dETS results by the effect of sulfate adsorption. (c) Schematic models of different Pt surface conditions in different electrochemical regions in HClO4 and with sulfate anions. Pt atoms are gray, H atoms are white, O atoms are red in H2Oads, blue in OHads, and green in Oads for a visual guide to the different scattering effects. Solid arrows in all figures indicate the potential sweeping direction, with corresponding dots showing the starting point of the measurement.