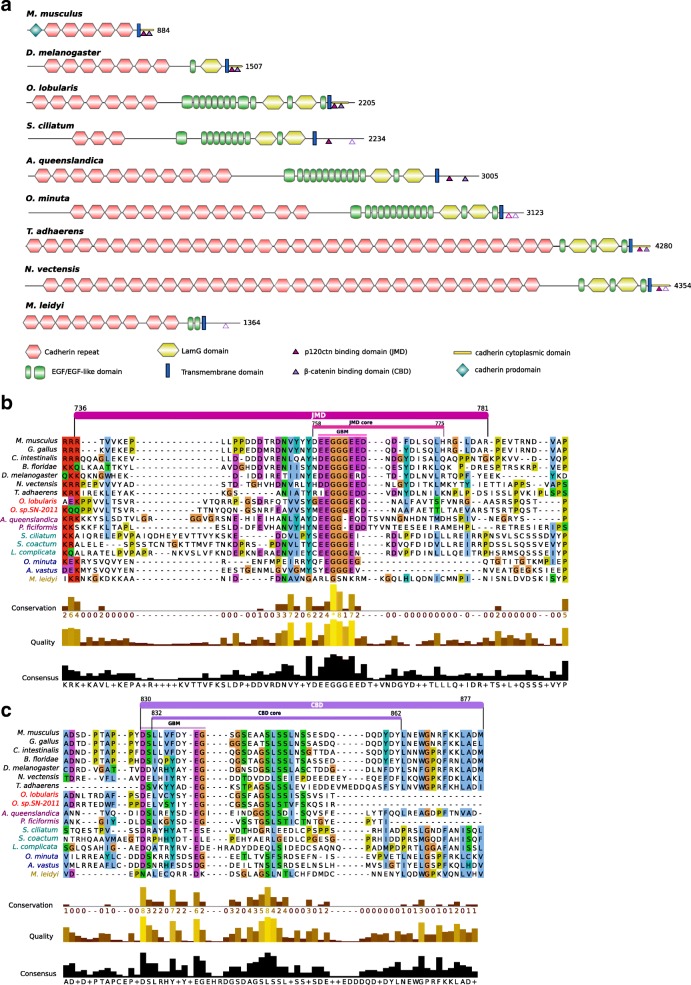
Fig. 1.
Comparison of E-cadherin domains and motifs between metazoans. Porifera: Homoscleromorpha in red (Oscarella lobularis, Oscarella. sp.), Demospongiae in magenta (Amphimedon queenslandica, Petrosia ficiformis), Calcarea in green (Sycon ciliatum, Sycon coactum, Leucosolenia complicata), Hexactinellida in blue (Oopsacas minuta, Aphrocallistes vastus). Other represented clades are Placozoa (Trichoplax adhaerens); Ctenophora (Mnemiopsis leidyi) in yellow, Cnidaria (Nematostella vectensis), Bilateria (Deuterostomia: Mus musculus; Protostomia: Drosophila melanogaster). Sequences were aligned with MAFFT v7 web server and visualized with Jalview. a Representative cadherin proteins depicted with their domains. Mus musculus and Drosophila melanogaster E-cadherins are taken as reference. Oscarella lobularis has the sole poriferan cadherin the cytoplasmic-specific domain of which is detected by Pfam (E-value = 2.10− 11) and InterProScan as in the mouse and fruitfly E-cadherin (depicted in yellow at the C-terminal part). Degrees of conservation of p120 and β-catenin binding domains are indicated by full, dashed or open triangles. b Alignment of the cytoplasmic cadherin p120 binding domain (Juxtamembrane domain, JMD). The JMD consists of 50 residues immediately following the transmembrane domain (in Mouse E-cadherin). The JMD core consists of 20 residues. The groove-binding motif (GBM) required for binding p120 is well conserved in metazoans. c Alignment of the cytoplasmic cadherin β-catenin binding domain (CBD). The CBD consists of approximately 50 residues. The groove-binding motif (GMB) consists of 10 residues