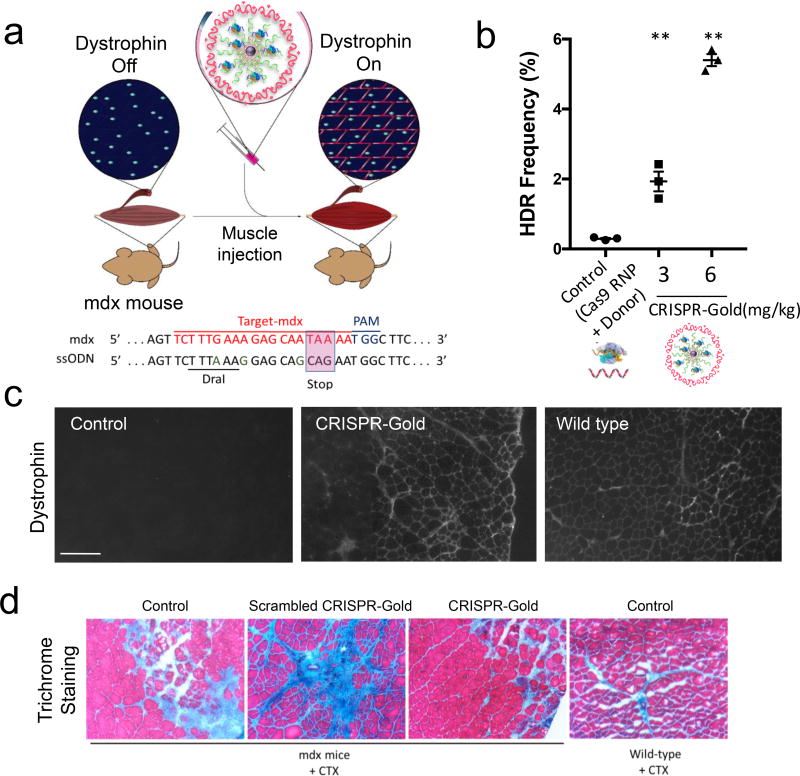
Figure 6. CRISPR-Gold promotes HDR in the dystrophin gene and dystrophin protein expression, and reduces muscle fibrosis in mdx mice, with CTX stimulation.
a) CRISPR-Gold was injected into the hind leg muscle of 8-week-old mdx mice (n=3), simultaneously with CTX. Two weeks later, the mice were sacrificed and analyzed for dystrophin expression by immunofluorescence, for HDR by deep sequencing and for their degree of fibrosis. Bottom panel: dystrophin mutation sequence and donor DNA design. Donor DNA sequence designed to repair the non-sense mutation are marked in the pink box, nucleotides marked in green (A, G, G) are silent mutations that prevent Cas9 activity on the edited sequence.
b) CRISPR-Gold induced genome editing in the dystrophin gene was confirmed by deep sequencing. Deep sequencing of genomic DNA from muscle tissues injected with CRISPR-Gold revealed a 5.4% HDR efficiency. The negative control composed of Cas9 RNP and donor DNA had an HDR efficiency of only 0.3%. Mean ± S.E, n=3. **, p < 0.01.
c) CRISPR-Gold causes dystrophin expression in muscle tissue of mdx mice. The CRISPR-Gold injected muscle shows dystrophin expression (immuno-fluorescence), whereas control mdx mice did not express dystrophin protein. The scale bar is 200 µm.
d) CRISPR-Gold reduces muscle fibrosis in mdx mice. Trichrome staining was performed on the TA muscle cryosectioned to 10 µm, 2 weeks after the injection of CRISPR-Gold. CTX was co-injected in all three groups of mdx mice: No treatment control, scrambled CRISPR-Gold, and CRISPR-Gold. Images were acquired at the areas of muscle injury and regeneration. Fibrotic tissue appears blue, while muscle fibers appear red. Wild-type mice treated with CTX were analyzed 5 days after injection. The width of each image is 0.7 mm.