Abstract
Background and objectives
Barriers exist in access to kidney transplantation, where minority and patients with low socioeconomic status are less likely to complete transplant evaluation. The purpose of this study was to examine the effectiveness of a transplant center–based patient navigator in helping patients at high risk of dropping out of the transplant evaluation process access the kidney transplant waiting list.
Design, setting, participants & measurements
We conducted a randomized, controlled trial of 401 patients (n=196 intervention and n=205 control) referred for kidney transplant evaluation (January 2013 to August 2014; followed through May 2016) at a single center. A trained navigator assisted intervention participants from referral to waitlisting decision to increase waitlisting (primary outcome) and decrease time from referral to waitlisting (secondary outcome). Time-dependent Cox proportional hazards models were used to determine differences in waitlisting between intervention and control patients.
Results
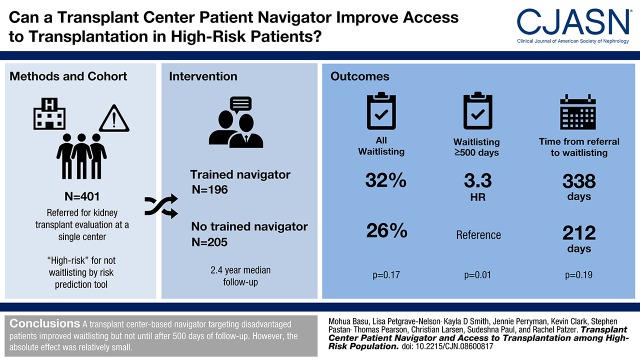
At study end, waitlisting was not significantly different among intervention (32%) versus control (26%) patients overall (P=0.17), and time from referral to waitlisting was 126 days longer for intervention patients. However, the effectiveness of the navigator varied from early (<500 days from referral) to late (≥500 days) follow-up. Although no difference in waitlisting was observed among intervention (50%) versus control (50%) patients in the early period (hazard ratio, 1.03; 95% confidence interval, 0.69 to 1.53), intervention patients were 3.3 times more likely to be waitlisted after 500 days (75% versus 25%; hazard ratio, 3.31; 95% confidence interval, 1.20 to 9.12). There were no significant differences in intervention versus control patients who started evaluation (85% versus 79%; P=0.11) or completed evaluation (58% versus 51%; P=0.14); however, intervention patients had more living donor inquiries (18% versus 10%; P=0.03).
Conclusions
A transplant center–based navigator targeting disadvantaged patients improved waitlisting but not until after 500 days of follow-up. However, the absolute effect was relatively small.
Keywords: clinical trial, transplant outcomes, risk factors, kidney transplantation, Humans, Living Donors, Patient Navigation, Proportional Hazards Models, Waiting Lists, Vulnerable Populations, Follow-Up Studies, kidney, Minority Groups, Transplants, Referral and Consultation, Social Class
Introduction
Multiple steps are required for a patient to receive a kidney transplant, including transplant education, referral for and starting the evaluation process at a transplant center, completion of medical and psychosocial assessments, and either identifying a living donor or being placed on the waiting list for a deceased donor transplant (1,2). There is the potential for delay in early steps of the transplant process (3–6), where racial and socioeconomic disparities exist in access to each transplant step (7–10).
Because of significant variability in time from referral to waitlisting, intervention at this early stage of the transplant process has potential to increase transplant access. Patient navigation has been a successful strategy in oncology and other fields to improve patient access (11–14). More recently, studies have shown that trained kidney transplant recipients serving as patient navigators for patients with ESKD within dialysis facilities doubled the number of steps that a patient completed in the transplant process (15) and increased access to living donor transplantation (16), but the effectiveness of a patient navigator program among a historically disadvantaged group of patients with ESKD has not been studied. The kidney transplant community has called for targeted interventions among disadvantaged groups as one approach to eliminate health disparities in transplantation (17–23); however, to reduce disparities, an intervention must be either more effective among the disparate group or implemented among the disparate group only (17).
The objective of this study was to determine if a patient navigator intervention in a large, urban southeastern transplant center was effective in improving access to the kidney transplant waiting list and reducing time from referral to waitlisting among patients referred to the transplant center. We focused on studying the effectiveness of this intervention on patients with the most need: a primarily socioeconomically disadvantaged patient population with the highest risk of dropping out of the transplant evaluation process that may have additional barriers, such as poverty, insurance coverage issues, medical comorbidities, lack of social support, and limited education about transplant (5,6,24–26), that could potentially be addressed by a patient navigator. We hypothesized that the navigator would increase waitlisting and reduce time from referral to waitlisting among patients who were medically suitable but who needed additional assistance to complete the required medical and psychosocial evaluation.
Materials and Methods
Study Population
From January 2013 to August 2014, all patients referred for kidney transplant evaluation at the transplant center were screened by phone by a transplant center staff at time of evaluation scheduling for potential inclusion in this randomized, controlled trial with a risk assessment tool predicting probability of waitlisting. This tool was built within a secure, Health Insurance Portability and Accountability Act-compliant REDCap database (27) and on the basis of a predictive model created using transplant center patient data (c statistic, 0.90; 95% confidence interval [95% CI], 0.89 to 0.91), incorporating characteristics associated with waitlisting, such as age, race, body mass index (BMI), educational level, marital status, etiology of ESKD, insurance status, primary language, and whether the patient was on dialysis at time of referral (Supplemental Table 1) (28). Researchers received a list of eligible patients (waitlisting risk score <40%) from transplant center staff and recruited patients via phone or in person before their evaluation appointment. Patients providing informed consent for the study were randomized by research study staff using a 1:1 randomized allocation table; the navigator and patient care team were not blinded to the intervention.
Study Procedures
Standard procedure at this transplant center is for patients to meet with a transplant nurse coordinator, social worker, financial coordinator, and physician’s assistant during the first day of evaluation. The intervention group received additional support from a trained patient navigator with a Master’s degree in social work who is black. The navigator was not part of the transplant team and worked with the intervention patients’ care team only as it related to study activities. The navigator called patients before their first evaluation appointment to conduct an initial assessment, met with patients during the evaluation appointment, and supported patients through waitlisting decision.
Navigator duties included transplant education with an emphasis on living donor transplant, because previous research has shown there are greater racial and socioeconomic disparities in living versus deceased donor transplant and living donation could reduce transplant waiting times (29–32); assisting with scheduling, appointment reminders, financial aid, transportation, and interpretative services for non-native English speakers; providing social support, including referrals to support groups and community resources; and serving as a liaison between the patient and other members of the patient’s care team and between the transplant center and outside health care and social service organizations, such as the patient’s dialysis facility. The navigator provided educational materials from the Emory Transplant Center and the National Kidney Fund, contact information for the Living Donor Hotline to promote living donor transplant, financial resources from the Georgia Transplant Foundation regarding financial assistance, Georgia Crisis Hotline information for patients with mental health issues, and pamphlets for weight loss, drug rehabilitation, or smoking cessation as needed. After each patient completed the evaluation, the navigator attended a multidisciplinary selection conference, where the patient was discussed by the care team and a waitlist decision was made. In addition, the navigator provided encouragement by calling the patient throughout the transplant process and/or meeting with the patient during subsequent appointments. Encouragement was intended to motivate patients to attend appointments and complete evaluation requirements.
The navigator logged each patient encounter from referral to waitlist decision, documenting the encounter by type (in person, phone, and email), who was contacted (patient or family member), the length of contact time, and reason for contact.
Study Variables
Patient demographic, clinical, and socioeconomic indicators from time of referral were captured in the REDCap database by transplant center clinical staff from either the patient referral form or when calling patients to schedule the first transplant evaluation appointment. Data were linked to electronic medical records to collect data on patient zip code, living donor inquiries, and dates of referral, evaluation, waitlisting, transplant, and death.
Demographic characteristics collected included race, ethnicity, sex, and age at time of referral. Clinical characteristics collected included BMI (<35 or ≥35 kg/m2, the cutoff for active waitlisting at the center), etiology of ESKD (diabetes, hypertension, or other), history of comorbidities (International Classification of Diseases-9 codes used to categorize hypertension, diabetes, cardiovascular disease, and cancer), previous transplant before referral, and length of time on dialysis at the time of referral. Socioeconomic characteristics included patient distance from transplant center (distance between zip codes), education, insurance, marital status, language, and probability of waitlisting on the basis of the risk prediction model, which was divided into three categories: low (<20%), moderate (20%–30%), and high (30%–40%).
Outcomes
The primary outcome was waitlisting for kidney transplant. The secondary outcome was the time from referral to waitlisting; we also examined time from referral to evaluation and time from evaluation to waitlisting among patients who started evaluation, and we examined time from referral to transplant among patients who received a transplant.
In addition, we explored whether the proportion of patients completing each of several transplant steps differed by study group, including completing transplant education (online-based education module recommended before evaluation start), starting transplant evaluation (arriving to day 1 of evaluation), completing transplant evaluation (if a waitlist decision was made by the transplant team after evaluation), receiving at least one living donor inquiry (candidate’s friend/family member called transplant center to inquire about testing), being placed on the deceased donor waitlist (active or inactive listing), and receiving a deceased or living donor transplant.
Statistical Analyses
Kaplan–Meier curves and Cox proportional hazards models were used to examine access to waitlist by study group using time from referral to waitlisting, censoring at death, or end of follow-up (May 1, 2016). Cox proportional hazards assumptions were examined using log-log curves, time by study group interaction, and Schoenfeld residuals. Because of violation of the proportional hazards assumption, we used time-dependent Cox models to examine waitlisting before and after 500 days, the point at which the Kaplan–Meier curves crossed. The proportional hazards assumptions were met for each time period (<500 and ≥500 days). For Kaplan–Meier curves, z statistics were calculated at 1, 2, and 3 years from referral.
Wilcoxon rank sum tests were used to examine differences by study group in time to study outcomes, because time intervals were not normally distributed. In exploratory analyses, chi-squared tests were used to examine differences in completion of each transplant step for intervention versus control patients. Logistic regression was used for exploratory analyses to determine if the number of encounters that the navigator had per patient was associated with study outcomes.
In our a priori power calculation on the basis of a prior study that found a more than doubling of waitlisting among intervention versus control participants (15), we initially aimed for a sample size of 800 patients. However, the target sample size was revised after recruitment began to accommodate the patient navigator’s work load; the new power calculation anticipated that a sample size of approximately 400 (200 in each group) would achieve 80% power to detect a hazard ratio (HR) of 1.6 comparing intervention patients with control patients, assuming a constant HR throughout the study, 30% rate of waitlisting among controls, and a 5% significance level.
SAS 9.4 was used for all statistical analyses, and two-tailed P<0.05 was considered statistically significant. This study was approved by the Emory Institutional Review Board and registered on ClinicalTrials.gov (NCT01776073). The clinical and research activities being reported are consistent with the Declaration of Helsinki and the Principles of the Declaration of Istanbul as outlined in the “Declaration of Istanbul on Organ Trafficking and Transplant Tourism.”
Results
Study Population
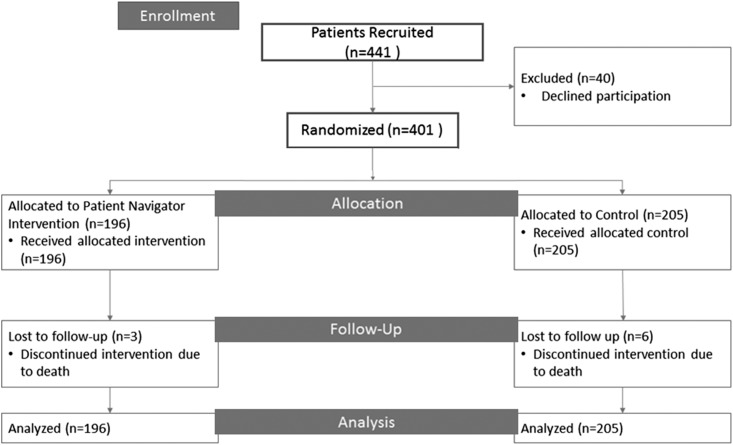
In the study period from January 2013 and August 2014, 947 patients were classified as “high risk” for not waitlisting by the REDCap risk prediction tool and thus, eligible for the study; researchers attempted to recruit from this list of patients until reaching the target sample size. Researchers contacted 441 patients to request participation in the study via phone or in person before the evaluation appointment, with 40 patients declining (91% response rate). A total of 401 patients were included in the final study population (196 intervention and 205 control patients) (Figure 1). During study follow-up, nine patients died (2% intervention versus 3% control) and were censored at the date of death.
Figure 1.
Flow diagram of study population of patients referred for kidney transplant evaluation to single-center transplant center from January 2013 to August 2014, of which 401 patients were randomized and included in analyses.
Patients in both the intervention and control groups had similar characteristics (Table 1). Overall, study participants had a median age of 54 years old (interquartile range [IQR], 45–64), and the majority were black (82%), were non-Hispanic (93%), had a history of hypertension (77%), had Medicare insurance (86%), and were not married (70%) at the time of referral. Approximately one third had not completed high school (32%) and had Medicaid (39%). There were more patients who were white, on dialysis for ≤2 years, and college educated in the control versus intervention group, but there were more patients of “other” race, of Hispanic ethnicity, who were non-native English speakers, with history of cardiovascular disease, and who lived closer to the transplant center in the intervention versus control group (Table 1).
Table 1.
Baseline characteristics of study participants at the time of referral for kidney transplant evaluation by study group from January 2013 to August 2014
| Patient Characteristics | Study Population, n=401 | Intervention, n=196 | Control, n=205 |
|---|---|---|---|
| Median age (IQR), yr | 54 (45–64) | 54 (46–63) | 54 (44–65) |
| Age categories, yr, N (%) | |||
| 18–39 | 62 (16) | 29 (15) | 33 (16) |
| 40–59 | 204 (51) | 102 (52) | 102 (50) |
| 60+ | 135 (34) | 65 (33) | 70 (34) |
| Men, N (%) | 205 (51) | 88 (45) | 117 (57) |
| Race, N (%) | |||
| White | 37 (9) | 13 (7) | 24 (12) |
| Black | 327 (82) | 159 (81) | 168 (82) |
| Other | 37 (9) | 24 (12) | 13 (6) |
| Ethnicity, N (%) | |||
| Hispanic | 25 (7) | 17 (9) | 8 (4) |
| Non-Hispanic | 351 (93) | 168 (91) | 183 (96) |
| Etiology of ESKD, N (%) | |||
| Diabetes | 140 (35) | 73 (37) | 67 (33) |
| Hypertension | 184 (46) | 88 (45) | 96 (47) |
| Other | 77 (19) | 35 (18) | 42 (21) |
| Body mass index, kg/m2, N (%) | |||
| <35 | 265 (66) | 133 (68) | 132 (64) |
| ≥35 | 136 (34) | 63 (32) | 73 (36) |
| Dialysis duration, N (%) | |||
| No dialysis | 25 (6) | 14 (7) | 11 (5) |
| ≤2 yr | 235 (59) | 111 (57) | 124 (61) |
| 2+yr | 141 (35) | 71 (36) | 70 (34) |
| Comorbidity history, N (%) | |||
| Diabetes | 186 (46) | 95 (49) | 91 (44) |
| Hypertension | 310 (77) | 155 (79) | 155 (76) |
| Cardiovascular disease | 255 (64) | 134 (68) | 121 (59) |
| Cancer | 41 (10) | 17 (9) | 24 (12) |
| Missing | 66 (17) | 30 (15) | 36 (18) |
| Prior transplant at referral, N (%) | 11 (3) | 6 (3) | 5 (2) |
| Education level, N (%) | |||
| Less than high school | 129 (32) | 64 (33) | 65 (32) |
| Completed high school | 150 (37) | 75 (38) | 75 (37) |
| Some college | 101 (25) | 50 (26) | 51 (25) |
| College or above | 21 (5) | 7 (3) | 14 (7) |
| Insurance status, N (%) | |||
| Medicare | 344 (86) | 163 (86) | 175 (85) |
| Medicaid | 156 (39) | 76 (39) | 80 (39) |
| Private | 39 (10) | 21 (11) | 18 (9) |
| None | 5 (1) | 3 (2) | 2 (1) |
| Primary language, N (%) | |||
| English | 376 (94) | 179 (91) | 197 (96) |
| Other | 25 (6) | 17 (9) | 8 (4) |
| Marital status, N (%) | |||
| Married | 121 (30) | 59 (30) | 62 (30) |
| Single | 280 (70) | 137 (70) | 143 (70) |
| Median distance (miles) to transplant center (IQR) | 26.1 (12.4–86.9) | 21.4 (12.0–81.0) | 30.7 (13.1–93.4) |
| Distance, miles, N (%) | |||
| <12 | 85 (21) | 49 (25) | 36 (18) |
| 12–25 | 111 (28) | 56 (29) | 55 (27) |
| 25–75 | 85 (21) | 36 (18) | 49 (24) |
| ≥75 | 120 (30) | 55 (28) | 65 (32) |
| Probability of waitlisting, %, N (%)a | |||
| <20 | 69 (17) | 37 (19) | 32 (16) |
| 20–30 | 132 (33) | 58 (30) | 74 (36) |
| 30–40 | 200 (50) | 101 (52) | 99 (48) |
IQR, interquartile range.
As determined by the REDCap risk prediction model applied at the time of referral.
Access to Waitlisting
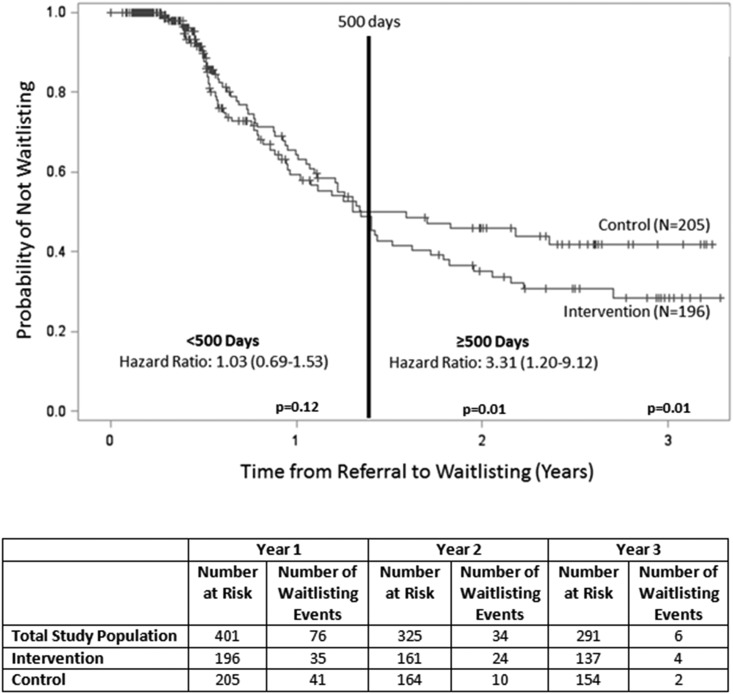
Over the median 2.4-year follow-up (IQR, 1.4–2.9) period through May 2016, 116 (29%) patients were waitlisted, including 63 (32%) intervention patients and 53 (26%) control patients (P=0.17) (Table 2). Among waitlisted patients in the study population, median time from referral to waitlisting was 282 days (IQR, 188–453); this was longer for intervention versus control patients (338 versus 212 days; P=0.19). A total of 96 waitlisting events (50% among intervention patients and 50% among control patients) occurred within the first 500 days, and 20 waitlisting events (75% among intervention patients and 25% among control patients) occurred after 500 days. In Cox models, no difference in waitlisting between intervention and control patients was observed within 500 days (HR, 1.03; 95% CI, 0.69 to 1.53). However, after 500 days, intervention patients were 3.3 times more likely to be waitlisted compared with control participants (HR, 3.3; 95% CI, 1.20 to 9.12) (Figure 2).
Table 2.
Effectiveness of patient navigation on completion of kidney transplant steps
| Transplant Steps | Study Population (%), n=401 | Intervention (%), n=196 | Control (%), n=205 | P Value |
|---|---|---|---|---|
| Start of transplant evaluation | 327 (82) | 166 (85) | 161 (79) | 0.11 |
| Completion of transplant education | 174 (43) | 89 (45) | 85 (42) | 0.22 |
| Completion of transplant evaluation | 218 (54) | 114 (58) | 104 (51) | 0.43 |
| Living donor inquiry (one or more) | 56 (14) | 35 (18) | 21 (10) | 0.03 |
| Placement on waitlist | 116 (29) | 63 (32) | 53 (26) | 0.34 |
| Receipt of transplant | 19 (5) | 9 (5) | 10 (5) | 0.89 |
Figure 2.
Kaplan–Meier analyses and time-dependent Cox proportional hazards model for waitlisting among intervention versus control study participants showed that patients with a navigator were more likely to be placed on the kidney transplant waiting list after 500 days.
Additionally, among 327 patients who started the evaluation, median time from referral to evaluation was 64 days (IQR, 51–84; intervention: 64 days [IQR, 52–84]; control: 63 days [IQR, 51–84]; P=0.88). Median time from evaluation to waitlist decision among the 327 patients starting the evaluation was 724 days (IQR, 315–940) overall (intervention: 721 days [IQR, 335–969]; control: 724 days [IQR, 306–919]; P=0.93). Median time from referral to transplant among 19 patients who received a transplant was 649 days (IQR, 461–739; intervention: 655 days [IQR, 588–850]; control: 579 days [IQR, 429–700]; P=0.25).
Exploratory Analyses
Although not powered to detect differences, intervention patients versus control patients were no more likely to start transplant evaluation (85% versus 79%; P=0.11), complete required education (45% versus 42%; P=0.43), or complete evaluation (58% versus 51%; P=0.14), but they were more likely to receive a living donor inquiry (18% versus 10%; P=0.03) (Table 2).
Navigator Encounters
The patient navigator made 1722 contacts among 196 patients, translating to approximately 340 direct patient-hours. The first contact with the patient was usually 15 minutes to 1 hour (54%) or <15 minutes (44%). Subsequent contacts were shorter: 88% of evaluation follow-up calls and 86% of reminders were <15 minutes. On average, the navigator contacted each patient eight times, primarily over the phone (89%) and with the patient alone (83%), although 16% of contacts also included a family member. Reasons for patient contact included referrals to relevant resources or organizations (52%), evaluation reminders (23%), evaluation follow-up (22%), scheduling assistance (16%), providing information on living donation (14%) and other transplant-related education (8%), weight loss education (5%), career- or education-related information (4%), and transportation assistance (4%). A greater number of navigator encounters per patient was associated with both starting (odds ratio [OR], 1.46; 95% CI, 1.24 to 1.71; P<0.001) and completing the evaluation (OR, 1.09; 95% CI, 1.03 to 1.16; P<0.01), completing the education module (OR, 1.06; 95% CI, 1.00 to 1.12; P=0.04), and waitlisting (OR, 1.12; 95% CI, 1.05 to 1.19; P≤0.001).
Discussion
In this study, we found that the effect of a transplant center–based patient navigator on waitlisting among a population of largely disadvantaged patients increased access to the waitlist but not until after 500 days of follow-up. After the first 500 days, patients with a navigator were over three times more likely to become waitlisted compared with patients without a navigator, but time from referral to waitlisting was 126 days longer for intervention versus control participants. This effect may be because this high-needs population may not have otherwise been waitlisted without a patient navigator but needed additional time to complete various medical tests and follow-up requirements compared with patients who waitlisted within the first 500 days. Although a navigator did not reduce time to waitlisting, the intervention could potentially still help a high-needs population successfully access the waitlist after 500 days by addressing frequently encountered barriers that may delay waitlisting. In addition, we found that patients assigned to a patient navigator had a higher number of living donor inquiries.
Two other studies have examined social worker or patient navigator interventions among patients with ESKD in dialysis centers (15,23). In a retrospective, single-center study, Marlow et al. (16) found that a social worker navigator in a community-based nephrology clinic increased living donor inquiries (OR, 1.31; 95% CI, 1.01 to 1.44), where approximately 40% of patients had at least one living donor inquiry. In our study, only 14% of patients had at least one living donor inquiry, but this was nearly double among the intervention versus control groups (18% versus 10%). Similarly, Sullivan et al. (15) found that the use of trained transplant recipient “lay” navigators within dialysis facilities doubled the total number of transplant steps completed among patients compared with the control group. However, that dialysis facility–based intervention was more intensive than our transplant-based intervention, because multiple navigators met with patients monthly over 2 years. Our strategy may not have been as successful in the first 500 days because of the use of only one navigator. In addition, Sullivan et al. (15) excluded patients at the dialysis facility who were >70 years old, had absolute contraindications to kidney transplant, and had communication barriers; unlike that study, our intervention targeted the most disadvantaged patients at our center, including patients with high BMI and non–English-speaking patients, for whom it may have been more challenging for a navigator to affect changes.
Our study is the first to our knowledge to use a patient navigator among patients at a transplant center rather than a dialysis facility and to target the intervention only to a high-risk group of majority black and lower-socioeconomic status patients. Previous research has shown that low-socioeconomic patients with ESKD have less access to transplant (6,33) and poorer health outcomes (34–36). To address these health disparities in transplant, interventions should be targeted to the disadvantaged population (17). In a prior study focusing on disadvantaged populations to improve kidney transplant access, health educators were randomized to visit black patients with ESKD and guests in their homes, which resulted in a trend toward increased living donor transplant and increased knowledge and willingness to discuss transplantation (37). Arriola et al. (38) also found that a culturally sensitive educational intervention at a transplant center increased knowledge and willingness to talk to patients’ families about transplant; however, key steps, such as waitlisting and transplant, were not examined.
There are several explanations for why we did not observe a difference in waitlisting among intervention patients versus control patients in the first 500 days of our study. First, it is possible that the navigator was overburdened with too many patients, especially because this patient population has greater needs than the typical patient population undergoing evaluation. Second, it is possible that, as time passed, the navigator became more experienced, being able to identify additional resources for patients and better assisting patients in meeting their complex needs. Third, because of our focus on patients at highest risk of dropping out of the transplant evaluation, it is possible that we included some patients with nonmodifiable barriers. Fourth, there was an approximately 6% higher proportion of intervention versus control patients who started the transplant evaluation who perhaps might have otherwise dropped out of the evaluation process, resulting in a 126-day longer time from referral to waitlisting; it is likely that these patients extended evaluation time for the intervention participants. Fifth, by chance alone, our study groups were somewhat unbalanced, with more minorities and non-English speakers who have historically had less access to transplant than their counterparts in the intervention group.
Findings should be considered in context with study limitations. First, we did not have preliminary data to estimate the effect size of our intervention a priori, and initial power calculations were on the basis of effect sizes observed from dialysis facility navigators in a prior study that did not focus on a disadvantaged population (15); thus, our study may have been underpowered. A sample size of 1400 over the same period of time powered at 80%, assuming a 30% rate of waitlisting among controls, a 0.05 significance level, and a constant HR would have been necessary to detect an HR of 1.3. Larger, multicenter studies testing the effectiveness of a patient navigator may be warranted, particularly because the absolute effect of the navigator was small after the 500 days (15 versus five waitlisting events). Perhaps future studies should administer multiple interventions along with patient navigation, such as educational interventions that take place in either a patient’s home (21) or the dialysis facility (39) and/or culturally sensitive education within a transplant center focusing on living donor transplant (38), to potentially have a synergistic effect on waitlisting and help address the major barriers faced by this population. Larger studies with longer follow-up could also examine important outcomes beyond waitlisting, including receipt of a living or deceased donor transplant. Second, not all patients were able to be contacted by the patient navigator before evaluation. Previous studies have found that the time between referral and evaluation is when many patients drop out of the process (3,4), and it may be a critical period in which patient navigation is helpful. Third, because the study was not blinded, other members of the patient care team may have treated patients differently, knowing that they had the additional support of a patient navigator. Fourth, results reflect a single-center experience and may not be generalizable to all centers. Our transplant center has among the highest number of black candidates in the nation, and our state has the lowest rate of kidney transplantation in the nation (40); therefore, results may be particularly relevant for the southeastern ESKD population. Fifth, we recognize that it is resource intensive for one navigator to tailor care for each patient and that it may not be sustainable. Finding the appropriate number of patients for a navigator may vary from center to center, and a lower navigator-to-patient ratio may be most beneficial in improving access to care. For example, we have previously reported that a lower social worker-to-patient ratio is associated with higher transplant referral in our state (41). Although upfront costs to the transplant center may be an issue, targeting the intervention to those patients most in need could help control transplant center costs. In addition, lay navigators have been found to be useful in previous studies (15,42–45) and may be a more cost-effective option to explore.
Study results suggest that using a social worker–trained patient navigator may help to increase waitlisting among a population of patients with ESKD at high risk of dropping out of the transplant process, especially after the first 500 days of follow-up. The substantial barriers encountered by this population may not be immediately modifiable by a single transplant center–based patient navigator; interventions may need to be combined with other effective education or outreach initiatives, both pretransplant and at the transplant center. However, in the long term, a patient navigator could have a positive effect on waitlisting among high-risk patients, even if patients require additional time in the transplant process.
Disclosures
None.
Supplementary Material
Acknowledgments
We thank Dawn Fletcher, Wanda Allison, Rachel Koval, Brendan Lovasik, and Perry Dykes for assistance with the study.
We thank the Carlos and Marguerite Mason Trust Fund for funding this study.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
See related Patient Voice and editorial, “An Evolving Continuum of Care for the Kidney Disease Patient Will Help the Transplant Center Patient Navigator,” and “What Else Can We Do to Ensure Transplant Equity for High-Risk Patients?,” on pages 519–520 and 529–530, respectively.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.08600817/-/DCSupplemental.
References
- 1.Kasiske BL, Cangro CB, Hariharan S, Hricik DE, Kerman RH, Roth D, Rush DN, Vazquez MA, Weir MR; American Society of Transplantation : The evaluation of renal transplantation candidates: Clinical practice guidelines. Am J Transplant 1[Suppl 2]: 3–95, 2001 [PubMed] [Google Scholar]
- 2.Gallon LG, Leventhal JR, Kaufman DB: Pretransplant evaluation of renal transplant candidates. Semin Nephrol 22: 515–525, 2002 [DOI] [PubMed] [Google Scholar]
- 3.Schold JD, Gregg JA, Harman JS, Hall AG, Patton PR, Meier-Kriesche HU: Barriers to evaluation and wait listing for kidney transplantation. Clin J Am Soc Nephrol 6: 1760–1767, 2011 [DOI] [PubMed] [Google Scholar]
- 4.Kucirka LM, Grams ME, Balhara KS, Jaar BG, Segev DL: Disparities in provision of transplant information affect access to kidney transplantation. Am J Transplant 12: 351–357, 2012 [DOI] [PubMed] [Google Scholar]
- 5.Monson RS, Kemerley P, Walczak D, Benedetti E, Oberholzer J, Danielson KK: Disparities in completion rates of the medical prerenal transplant evaluation by race or ethnicity and gender. Transplantation 99: 236–242, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weng FL, Joffe MM, Feldman HI, Mange KC: Rates of completion of the medical evaluation for renal transplantation. Am J Kidney Dis 46: 734–745, 2005 [DOI] [PubMed] [Google Scholar]
- 7.Patzer RE, Amaral S, Wasse H, Volkova N, Kleinbaum D, McClellan WM: Neighborhood poverty and racial disparities in kidney transplant waitlisting. J Am Soc Nephrol 20: 1333–1340, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Patzer RE, McClellan WM: Influence of race, ethnicity and socioeconomic status on kidney disease. Nat Rev Nephrol 8: 533–541, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saunders MR, Lee H, Alexander GC, Tak HJ, Thistlethwaite JR Jr, Ross LF: Racial disparities in reaching the renal transplant waitlist: Is geography as important as race? Clin Transplant 29: 531–538, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Purnell TS, Hall YN, Boulware LE: Understanding and overcoming barriers to living kidney donation among racial and ethnic minorities in the United States. Adv Chronic Kidney Dis 19: 244–251, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jean-Pierre P, Hendren S, Fiscella K, Loader S, Rousseau S, Schwartzbauer B, Sanders M, Carroll J, Epstein R: Understanding the processes of patient navigation to reduce disparities in cancer care: Perspectives of trained navigators from the field. J Cancer Educ 26: 111–120, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parker VA, Clark JA, Leyson J, Calhoun E, Carroll JK, Freund KM, Battaglia TA: Patient navigation: Development of a protocol for describing what navigators do. Health Serv Res 45: 514–531, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Paskett ED, Harrop JP, Wells KJ: Patient navigation: An update on the state of the science. CA Cancer J Clin 61: 237–249, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Parker VA, Lemak CH: Navigating patient navigation: Crossing health services research and clinical boundaries. Adv Health Care Manag 11: 149–183, 2011 [DOI] [PubMed] [Google Scholar]
- 15.Sullivan C, Leon JB, Sayre SS, Marbury M, Ivers M, Pencak JA, Bodziak KA, Hricik DE, Morrison EJ, Albert JM, Navaneethan SD, Reyes CM, Sehgal AR: Impact of navigators on completion of steps in the kidney transplant process: A randomized, controlled trial. Clin J Am Soc Nephrol 7: 1639–1645, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marlow NM, Kazley AS, Chavin KD, Simpson KN, Balliet W, Baliga PK: A patient navigator and education program for increasing potential living donors: A comparative observational study. Clin Transplant 30: 619–627, 2016 [DOI] [PubMed] [Google Scholar]
- 17.Patzer RE, Pastan SO: Measuring the disparity gap: Quality improvement to eliminate health disparities in kidney transplantation. Am J Transplant 13: 247–248, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Williams AW: Health policy, disparities, and the kidney. Adv Chronic Kidney Dis 22: 54–59, 2015 [DOI] [PubMed] [Google Scholar]
- 19.Rodrigue JR, Kazley AS, Mandelbrot DA, Hays R, LaPointe Rudow D, Baliga P; American Society of Transplantation : Living donor kidney transplantation: Overcoming disparities in live kidney donation in the US--recommendations from a consensus conference. Clin J Am Soc Nephrol 10: 1687–1695, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.LaPointe Rudow D, Hays R, Baliga P, Cohen DJ, Cooper M, Danovitch GM, Dew MA, Gordon EJ, Mandelbrot DA, McGuire S, Milton J, Moore DR, Morgievich M, Schold JD, Segev DL, Serur D, Steiner RW, Tan JC, Waterman AD, Zavala EY, Rodrigue JR: Consensus conference on best practices in live kidney donation: Recommendations to optimize education, access, and care. Am J Transplant 15: 914–922, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Waterman AD, McSorley AM, Peipert JD, Goalby CJ, Peace LJ, Lutz PA, Thein JL: Explore Transplant at Home: A randomized control trial of an educational intervention to increase transplant knowledge for Black and White socioeconomically disadvantaged dialysis patients. BMC Nephrol 16: 150, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gordon EJ, Feinglass J, Carney P, Vera K, Olivero M, Black A, O’Connor KG, Baumgart JM, Caicedo JC: A website intervention to increase knowledge about living kidney donation and transplantation among hispanic/Latino dialysis patients. Prog Transplant 26: 82–91, 2016 [DOI] [PubMed] [Google Scholar]
- 23.Boulware LE, Hill-Briggs F, Kraus ES, Melancon JK, Falcone B, Ephraim PL, Jaar BG, Gimenez L, Choi M, Senga M, Kolotos M, Lewis-Boyer L, Cook C, Light L, DePasquale N, Noletto T, Powe NR: Effectiveness of educational and social worker interventions to activate patients’ discussion and pursuit of preemptive living donor kidney transplantation: A randomized controlled trial. Am J Kidney Dis 61: 476–486, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ayanian JZ, Cleary PD, Weissman JS, Epstein AM: The effect of patients’ preferences on racial differences in access to renal transplantation. N Engl J Med 341: 1661–1669, 1999 [DOI] [PubMed] [Google Scholar]
- 25.Traino HM, Nonterah CW, Cyrus JW, Gillespie A, Urbanski M, Adair-Kriz M: Disparities in the completion of steps to kidney transplantation: Protocol for a systematic review. BMJ Open 5: e008677, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Siskind E, Alex A, Alexander M, Akerman M, Mathew C, Fishbane L, Thomas J, Israel E, Fana M, Evans C, Godwin A, Agorastos S, Mellace B, Rosado J, Rajendran PP, Krishnan P, Ramadas P, Flecha A, Kiernan L, Morgan RM, Ali N, Sachdeva M, Calderon K, Hong S, Kaur J, Basu A, Nicastro J, Coppa G, Bhaskaran M, Molmenti E: Factors associated with completion of pre-kidney transplant evaluations. Int J Angiol 23: 23–28, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG: Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 42: 377–381, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Basu M, Clark K, Perryman J, Fletcher D, Allison W, Petgrave-Nelson L, Dykes P, Patzer RE: Development and application of a risk assessment tool for patients referred for kidney transplantation. Am J Transplant 14[Suppl S3]: 655, 2014 [Google Scholar]
- 29.Arriola K, Powell L, Thompson N, Perryman J: Living donor transplant education for AA ESRD patients. Presented at the APHA Annual Meeting, Boston, MA, November 2–6, 2013 [Google Scholar]
- 30.Weng FL, Reese PP, Mulgaonkar S, Patel AM: Barriers to living donor kidney transplantation among black or older transplant candidates. Clin J Am Soc Nephrol 5: 2338–2347, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lunsford SL, Simpson KS, Chavin KD, Menching KJ, Miles LG, Shilling LM, Smalls GR, Baliga PK: Racial disparities in living kidney donation: Is there a lack of willing donors or an excess of medically unsuitable candidates? Transplantation 82: 876–881, 2006 [DOI] [PubMed] [Google Scholar]
- 32.Gore JL, Danovitch GM, Litwin MS, Pham P-T, Singer JS: Disparities in the utilization of live donor renal transplantation. Am J Transplant 9: 1124–1133, 2009 [DOI] [PubMed] [Google Scholar]
- 33.Ashby VB, Kalbfleisch JD, Wolfe RA, Lin MJ, Port FK, Leichtman AB: Geographic variability in access to primary kidney transplantation in the United States, 1996-2005. Am J Transplant 7: 1412–1423, 2007 [DOI] [PubMed] [Google Scholar]
- 34.Morton RL, Schlackow I, Mihaylova B, Staplin ND, Gray A, Cass A: The impact of social disadvantage in moderate-to-severe chronic kidney disease: An equity-focused systematic review. Nephrol Dial Transplant 31: 46–56, 2016 [DOI] [PubMed] [Google Scholar]
- 35.Schaeffner ES, Mehta J, Winkelmayer WC: Educational level as a determinant of access to and outcomes after kidney transplantation in the United States. Am J Kidney Dis 51: 811–818, 2008 [DOI] [PubMed] [Google Scholar]
- 36.Goldfarb-Rumyantzev AS, Sandhu GS, Baird B, Barenbaum A, Yoon JH, Dimitri N, Koford JK, Shihab F: Effect of education on racial disparities in access to kidney transplantation. Clin Transplant 26: 74–81, 2012 [DOI] [PubMed] [Google Scholar]
- 37.Rodrigue JR, Paek MJ, Egbuna O, Waterman AD, Schold JD, Pavlakis M, Mandelbrot DA: Making house calls increases living donor inquiries and evaluations for blacks on the kidney transplant waiting list. Transplantation 98: 979–986, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Arriola KR, Powell CL, Thompson NJ, Perryman JP, Basu M: Living donor transplant education for African American patients with end-stage renal disease. Prog Transplant 24: 362–370, 2014 [DOI] [PubMed] [Google Scholar]
- 39.Patzer RE, Paul S, Plantinga L, Gander J, Sauls L, Krisher J, Mulloy LL, Gibney EM, Browne T, Zayas CF, McClellan WM, Arriola KJ, Pastan SO; Southeastern Kidney Transplant Coalition : A randomized trial to reduce disparities in referral for transplant evaluation. J Am Soc Nephrol 28: 935–942, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Patzer RE, Plantinga L, Krisher J, Pastan SO: Dialysis facility and network factors associated with low kidney transplantation rates among United States dialysis facilities. Am J Transplant 14: 1562–1572, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Patzer RE, Plantinga LC, Paul S, Gander J, Krisher J, Sauls L, Gibney EM, Mulloy L, Pastan SO: Variation in dialysis facility referral for kidney transplantation among patients with end-stage renal disease in Georgia. JAMA 314: 582–594, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Robie L, Alexandru D, Bota DA: The use of patient navigators to improve cancer care for Hispanic patients. Clin Med Insights Oncol 5: 1–7, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Steinberg ML, Fremont A, Khan DC, Huang D, Knapp H, Karaman D, Forge N, Andre K, Chaiken LM, Streeter OE Jr.: Lay patient navigator program implementation for equal access to cancer care and clinical trials: Essential steps and initial challenges. Cancer 107: 2669–2677, 2006 [DOI] [PubMed] [Google Scholar]
- 44.Raich PC, Whitley EM, Thorland W, Valverde P, Fairclough D; Denver Patient Navigation Research Program : Patient navigation improves cancer diagnostic resolution: An individually randomized clinical trial in an underserved population. Cancer Epidemiol Biomarkers Prev 21: 1629–1638, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cartmell KB, Bonilha HS, Matson T, Bryant DC, Zapka JG, Bentz TA, Ford ME, Hughes-Halbert C, Simpson KN, Alberg AJ: Patient participation in cancer clinical trials: A pilot test of lay navigation. Contemp Clin Trials Commun 3: 86–93, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.