Abstract
Background/Aims
The incidence of morbid obesity has exponentially increased over the last decades. Bariatric surgery (BS) has been proven effective in inducing weight loss and resolving comorbidities associated with morbid obesity. However, BS can also lead to major diagnostic and treatment challenges in patients who develop upper gastrointestinal malignancies. It is important to create awareness of this rising problem.
Methods
Relevant literature was searched in PubMed.
Results
(Formerly) obese patients are more prone to develop upper gastrointestinal malignancies, mainly adenocarcinoma of the distal esophagus, since obesity induces a chronic pro-inflammatory state due to endocrinological changes. When an upper gastrointestinal malignancy develops after BS, diagnosis is often delayed and challenging due to a different presentation of complaints and the altered anatomy following the earlier surgery. Also, a potentially curative resection is often more complex and reconstruction of the gastrointestinal continuity can be seriously hampered.
Conclusion
Due to the growing incidence of obesity and the increasing number of bariatric surgical procedures that are performed each year, it is expected that over the years to come, more post-BS patients will be diagnosed with upper gastrointestinal malignancies, providing great diagnostic and treatment challenges. Clinicians should be aware of this rising problem.
Keywords: Upper gastrointestinal malignancies, Esophageal cancer, Gastric cancer, Bariatric surgery, Upper gastrointestinal surgery
Introduction
The incidence of morbid obesity around the world has exponentially increased over the last decades [1]. According to the WHO, in 2016, more than 1.9 billion adults were overweight of whom 600 million were obese. Worldwide 2.8 million adults die each year following complications of obesity. In the fight against this epidemic, there has also been a steep increase in the number of bariatric surgical procedures, such as the sleeve gastrectomy (Fig. 1a) or gastric bypass (Fig. 1b). In morbidly obese patients, bariatric surgery (BS) has been proven effective, not only in inducing weight loss, but also in resolving comorbidities such as diabetes mellitus and hypertension [2].
Fig. 1.
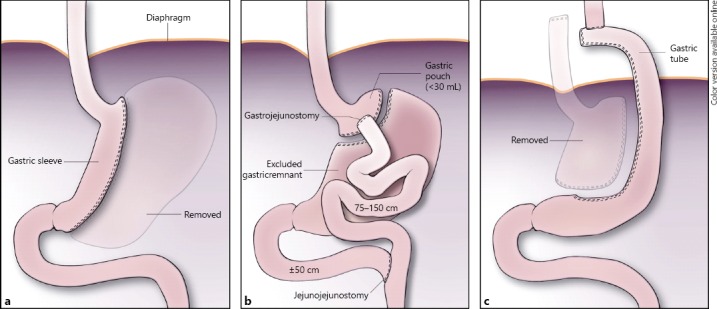
Schematic representation of state following gastric sleeve resection (resected greater curvature is printed dimly, a); b state following gastric bypass surgery; c state following gastric tube reconstruction.
Despite these clear advantages, BS also has some disadvantages, which can lead to major diagnostic and treatment challenges. A frequently seen difficulty is, for example, the regular need for vitamin supplementation because of malabsorption due to the bypassed and functionally shorter bowel. Furthermore, the interpretation of non-specific abdominal complaints becomes more difficult because of the great anatomical and physiological changes. Moreover, in case of the more frequently encountered bile duct stones, an endoscopic retrograde cholangiopancreatography becomes challenging [3]. Finally, a less frequent, but particularly difficult problem arises when an upper gastrointestinal malignancy develops in these patients.
In recent years, we have encountered an increase in post-BS patients presenting with upper gastrointestinal malignancies, confronting us with specific diagnostic and treatment challenges. Since BS is gaining more popularity each year, it is expected that the incidence of upper gastrointestinal malignancies in post-BS patients will show a steep increase as well. The specific challenges in diagnosis and treatment are described below, which clinicians will increasingly encounter in patients undergoing BS.
Obesity and Its Carcinogenic Potential
Obesity induces a chronic pro-inflammatory state due to endocrine changes such as increased leptin and decreased adipokine release by adipocytes. Obesity is the driving force behind the metabolic syndrome, characterized by (central) obesity, insulin resistance, dyslipidemia, and hypertension [1, 4, 5, 6]. This condition, also known as the “deadly quartet,” increases the risk of developing diabetes mellitus and cardiovascular diseases, but also has a (systemic) carcinogenic effect resulting in an increased risk of developing various types of cancer, with esophageal adenocarcinoma leading this list [3, 5, 6]. It has been shown that following BS, the risk of developing a variety of different tumors decreases again, further indicating that this relationship between obesity and carcinogenesis is indeed causal [4]. However, the incidence of malignancies in formerly obese patients after BS remains higher than in the general population.
A body mass index of ≥30 kg/m2 quadruples the risk of developing an esophageal adenocarcinoma compared to those with body mass index <25 kg/m2. Although the exact underlying mechanisms are not yet completely understood, various hypotheses have been proposed. Besides the systemic pro-inflammatory carcinogenic effect of obesity, one of the local contributing factors is the increased incidence of gastroesophageal reflux disease, leading to erosive esophagitis and ultimately Barrett esophagus, which is the premalignant condition for esophageal adenocarcinoma [7]. This higher incidence of gastroesophageal reflux disease is probably caused by increased intra-abdominal pressure, delayed gastric emptying, decreased lower esophageal sphincter pressure, and increased transient lower esophageal sphincter relaxations, which have all been described in obese patients [1].
Diagnostic Challenges
When an upper gastrointestinal malignancy develops after BS, diagnosis in these patients is often challenging. Vague symptoms, such as mild dysphagia and abdominal pain, are the first and often only signs [3]. Particular diagnostic challenge occurs when a malignancy develops in the excluded gastric remnant following gastric bypass surgery. Initially the tumor remains silent and symptoms appear relatively late. Because of its late and altered presentation, diagnosis is often delayed resulting in a more advanced stage of tumor at the time of diagnosis. Double balloon retrograde endoscopy performed via the Roux-en-Y anastomosis or antegrade endoscopy via laparoscopically assisted gastrotomy may be necessary to reach the excluded gastric remnant in order to examine the gastric mucosa and to take biopsies for proper diagnosis [3]. Due to the late presentation and difficult diagnostic work-up, less patients are eligible for potentially curative surgery [3].
Because of its malignant potential, the presence of Barrett's tissue in the distal esophagus is a contraindication to perform a gastric sleeve for weight loss. A recent review article by Braghetto and Csendes [8] showed that a Roux-en-Y gastric bypass significantly improves reflux symptoms and can provide regression of intestinal metaplasia of the distal esophageal mucosa. In case of esophageal or gastric cancer, curative options should be the primary goal. In order to diagnose Barrett's tissue or possible malignancies prior to BS, the European guidelines for BS advise an endoscopy prior to considering BS [9, 10].
Due to the increased malignant potential in (formerly) obese patients, lifelong frequent endoscopic monitoring is also advised [9]. For structures that are assessed with difficulty by endoscopy, such as the gastric remnant following a gastric bypass, CT may be considered for monitoring, although this diagnostic modality is not as sensitive to detect early lesions.
Hampered Oncological Surgery
If stage permits, potentially curative resection is often challenging due to the altered anatomy following BS. In case of gastric cancer, oncological resection of the stomach and surrounding lymph nodes is technically more demanding. In patients with esophageal or esophago-gastric junctional cancer, reconstruction of gastrointestinal continuity is seriously hampered. In these cases, endoscopic resection of early tumor is the preferred option, however, in most cases this is not possible due to the more advanced tumor stage at time of diagnosis because of the lack or vagueness of symptoms patients may experience after BS.
After having undergone a previous sleeve gastrectomy (Fig. 1a), conventional reconstruction with a gastric tube (made of the greater curvature; Fig. 1c) is no longer possible. In such cases, a high intrathoracic esophago-jejunostomy, if possible, and a colonic interposition are the reconstructive options, accompanied by higher percentages of postoperative complications, especially anastomotic leakage. Vascularization may be compromised in case of high esophago-jejunostomy, while 4 anastomoses are to be created in the case of colonic interposition.
In patients with a gastric bypass, the distal part of the stomach, duodenum, and first part of the small bowel are bypassed (Fig. 1b). In case of esophageal carcinoma, a gastric tube can generally still be created from the gastric remnant; however, it is also necessary to restore the normal anatomy of the jejuno-jejunostomy under these circumstances to prevent a short-bowel syndrome postoperatively.
Conclusion
Despite the clear advantages of BS, clinicians are confronted with serious diagnostic and treatment challenges when an upper gastrointestinal malignancy develops. Due to the increased carcinogenic potential in obese patients (especially for developing esophageal adenocarcinoma), the ever growing incidence of obesity and the rapidly increasing number of BS procedures, it is expected that more post-BS patients will be diagnosed over time with upper gastrointestinal malignancies. These patients often suffer from more advanced tumors at the time of first presentation because of the altered anatomy, confronting the clinicians with diagnostic challenges since the tumor is often difficult, if not impossible, to be reached by endoscopy. If tumor stage permits, surgery in these patients is more challenging. In case of gastric cancer, oncological resection is technically more complex, while in patients with esophageal and junctional cancer, reconstruction of gastrointestinal continuity is more demanding, requiring a high esophago-jejunal anastomosis or colonic interposition, both at an increased risk of postoperative complications. Gastroenterologists and surgeons should be aware of this rising problem.
Disclosure Statement
The authors declare that they have no conflicts of interest.
Funding Sources
The authors declare that they have no support of funding sources.
References
- 1.Ryan AM, et al. Barrett esophagus: prevalence of central adiposity, metabolic syndrome, and a proinflammatory state. Ann Surg. 2008;247:909–915. doi: 10.1097/SLA.0b013e3181612cac. [DOI] [PubMed] [Google Scholar]
- 2.Sjostrom L, et al. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med. 2004;351:2683–2693. doi: 10.1056/NEJMoa035622. [DOI] [PubMed] [Google Scholar]
- 3.Scozzari G, et al. Esophagogastric cancer after bariatric surgery: systematic review of the literature. Surg Obes Relat Dis. 2013;9:133–142. doi: 10.1016/j.soard.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 4.Sjostrom L, et al. Effects of bariatric surgery on cancer incidence in obese patients in Sweden (Swedish Obese Subjects Study): a prospective, controlled intervention trial. Lancet Oncol. 2009;10:653–662. doi: 10.1016/S1470-2045(09)70159-7. [DOI] [PubMed] [Google Scholar]
- 5.Cornier MA, et al. The metabolic syndrome. Endocr Rev. 2008;29:777–822. doi: 10.1210/er.2008-0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Renehan AG, et al. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371:569–578. doi: 10.1016/S0140-6736(08)60269-X. [DOI] [PubMed] [Google Scholar]
- 7.Hampel H, Abraham NS, El-Serag HB. Meta-analysis: obesity and the risk for gastroesophageal reflux disease and its complications. Ann Intern Med. 2005;143:199–211. doi: 10.7326/0003-4819-143-3-200508020-00006. [DOI] [PubMed] [Google Scholar]
- 8.Braghetto I, Csendes A. Patients having bariatric surgery: surgical options in morbidly obese patients with Barrett's esophagus. Obes Surg. 2016;26:1622–1626. doi: 10.1007/s11695-016-2198-9. [DOI] [PubMed] [Google Scholar]
- 9.Sauerland S, et al. Obesity surgery: evidence-based guidelines of the European Association for Endoscopic Surgery (EAES) Surg Endosc. 2005;19:200–221. doi: 10.1007/s00464-004-9194-1. [DOI] [PubMed] [Google Scholar]
- 10.Fried M, et al. Interdisciplinary European guidelines on surgery of severe obesity. Obes Facts. 2008;1:52–59. doi: 10.1159/000113937. [DOI] [PMC free article] [PubMed] [Google Scholar]


