Scheme 1.
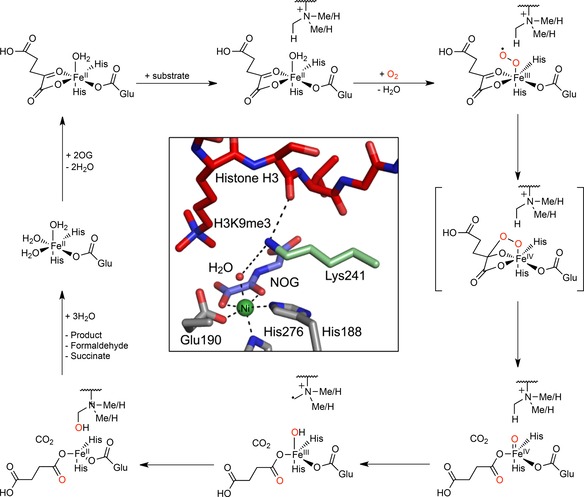
Outline mechanism of KDM4A‐catalysed demethylation. After binding of 2OG to the ferrous iron at the active site, the histone substrate binds. O2 then coordinates to the iron, thereby initiating an oxidative decarboxylation reaction, forming succinate, CO2 and a reactive iron(IV)‐oxo intermediate. Insertion of the iron(IV)‐bound oxygen atom into a histone methyl C−H bond occurs, with resultant reduction of the iron(IV) to iron(II). The hemiaminal product on the histone then fragments, giving the demethylated product and formaldehyde. Inset. A view of an X‐ray crystal structure of KDM4A complexed with Ni (substituting for FeII, green)), N‐oxalylglycine (NOG; a 2OG analogue, blue) and a histone H3 fragment peptide N ϵ‐trimethylated at Lys9 (red). Lys241 is shown in pale green (PDB ID: 2OQ6).
