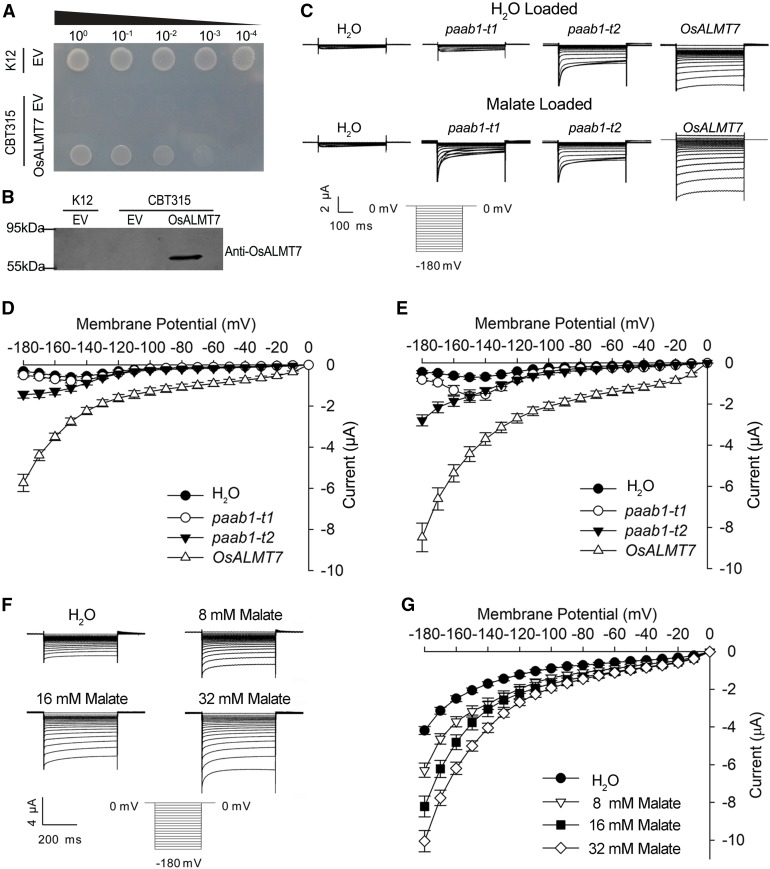
Figure 6.
sALMT7 Mediates Malate Transport in E. coli and X. laevis Oocytes Systems.
(A) and (B) Partial suppression of the growth defect of the E. coli dicarboxylate transporter mutant CBT315 by expression of OsALMT7. CBT315 transfected with the empty vector (EV) or the OsALMT7 gene (OsALMT7) and its wild type (K12) transfected with the EV only were grown on M9 agar medium with 10 mM malate (pH 6.6) as the sole carbon source. From left to right: transfected cells without dilution (100), diluted by 10× (10−1), 100× (10−2), 1000× (10−3), or 10,000× (10−4). Pictures were taken 3 d after inoculation. Immunoblot analysis showing the expression of the OsALMT7 protein in CBT315 is in (B).
(C) Current recordings in X. laevis oocytes expressing OsALMT7 and the paab1-1 mutants. Whole-cell currents were recorded in oocytes injected with different cRNAs: OsALMT7, paab1-t1, paab1-t2, and with water as a control. The oocytes were preloaded with either water (upper panel) or malate (bottom panel). The holding potential was set to 0 mV and voltage protocols, as well as time and current scale bars for the recordings, are shown. paab1-t1 and paab1-t2 represent two cDNAs corresponding the two mutant versions of transcripts detected in paab1-1.
(D) and (E) The current-voltage (I-V) relationship of the steady state currents in oocytes preloaded with water (D) or malate (E). The data are derived from the current recordings as those shown in (C) and presented as mean ± se (n = 10 for each cRNA).
(F) OsALMT7-mediated currents recorded from X. laevis oocytes preloaded with a range of malate concentrations. Whole-cell currents were recorded in oocytes expressing OsALMT7 at different intracellular malate concentrations (8, 16, and 32 mM). The oocytes preloaded with water were used as a control. The holding potential was set to 0 mV and voltage pluses were stepped between −180 mV and 0 mV in 10-mV increments. The time and current scale bars for the recordings are shown.
(G) The current-voltage (I-V) relationship of the steady state currents in oocytes with different intracellular malate concentrations. The data are derived from the current recordings as those shown in (F) and presented as mean ± se (n = 10 for each malate concentration).