Abstract
The aim of this study was to review fetoscopic laser photocoagulation (FLP), which ablates placental vascular anastomoses to treat twin–twin transfusion syndrome (TTTS). A review of studies reporting on the procedures, outcomes, complications and nonconventional applications of FLP for TTTS was conducted. FLP has been established as the primary treatment for monochorionic twin pregnancy associated with TTTS at 16–26 weeks. FLP is the only therapy that directly addresses the underlying pathophysiology. The recent technique modification of FLP, referred to as the ‘Solomon technique’, induces selective coagulation to connect the anastomoses ablation sites and has been introduced to reduce residual anastomoses. The perinatal survival following FLP improved significantly with advances in the technique after its introduction. The recent survival rates of both twins and at least one twin are 70% and more than 90%, respectively. However, there is still an 11–14% risk of long‐term neurodevelopment impairment. The premature rupture of membranes that leads to preterm labor is a common complication after FLP. FLP is a valuable treatment option for feto‐fetal transfusion syndrome in triplets and for TTTS after 26 weeks. FLP for selective intrauterine growth restriction may be potentially beneficial when accompanied by abnormal Doppler findings and oligohydramnios. FLP is the optimal treatment option for TTTS at 16–26 weeks of gestation. FLP appears to be applicable in triplets, TTTS after 26 weeks and cases of selective intrauterine growth restriction with abnormal Doppler findings and oligohydramnios. FLP is the most common and successful fetal intervention. Improvement in the neurodevelopmental outcomes after FLP is a future focus.
Keywords: fetal therapies, feto‐fetal transfusion, fetoscope, laser therapy, twins
Introduction
Twin–twin transfusion syndrome (TTTS), which affects 10% of monochorionic (MC) twin pregnancies, results in high perinatal morbidity and mortality.1, 2 MC twins share the same placenta, and vascular anastomoses allow blood to flow between the two fetuses. The etiology of TTTS is thought to be a hemodynamic and probably hormonal discordance secondary to a chronic blood flow imbalance between twins through placental vascular anastomoses.3, 4 Fetoscopic laser photocoagulation (FLP), which occludes placental vascular anastomoses, is thought to be useful for treating TTTS by directly coping with the underlying pathophysiology. The original technique of FLP was first described by De Lia et al. with laparotomy5 and developed by Ville et al. as a minimally invasive, percutaneous approach to be performed under ultrasound guidance.6, 7 Retrospective studies have noted that FLP resulted in high survival rates and low rates of neurological complications.7, 8, 9 The prospective randomized trial by the Eurofoetus group demonstrated that FLP is a superior and more effective first‐line treatment than serial amnioreduction.10 A meta‐analysis also showed that FLP is associated with better outcomes than serial amnioreduction.11 FLP is widely accepted and routinely offered for TTTS to improve the survival rate and neurological outcomes in fetal treatment centers across the world.
The purpose of this review was to update the knowledge regarding FLP relating to our clinical practice. We reviewed the procedures, outcomes and complications of FLP for TTTS and outlined the nonconventional applications of FLP and the status of FLP in Japan.
Staging of TTTS
TTTS is diagnosed on cases of MC twin pregnancy complicated by polyhydramnios with a maximum vertical pocket (MVP) ≥8.0 cm (with a distended bladder) in the recipient and oligohydramnios with an MVP ≤2.0 cm (with a nondistended bladder) in the donor. The Quintero staging system based on ultrasound findings (bladder invisibility, Doppler findings, hydrops and demise of the fetus) is simple and remains the most commonly used classification for TTTS.12 Murakoshi et al. subclassified Quintero stage 3 into classical and atypical cases based on the visibility of the donor bladder.13 Stage 3 atypical cases that show abnormal Doppler findings with a visible bladder have a significantly higher incidence of arterioarterial anastomoses and intrauterine fetal demise than classical cases. However, although the Quintero staging system is useful for describing the severity of the disease, it does not reflect the progression of the disease. Chronic blood flow imbalance via vascular anastomoses is understood to cause a fetal cardiovascular loading condition.14 Fetal echocardiographic changes are used to modify the staging system. The Children's Hospital of Philadelphia cardiovascular score and Cincinnati staging system have been proposed. 15, 16 However, these scores have not proven to be significantly useful in clinical settings.
FLP procedure
The procedure is illustrated in Figure 1. FLP uses intrauterine fetoscopy with a laser fiber. Under adequate anesthesia (regional or local), a small skin incision is performed. A 3.8‐mm cannula is percutaneously inserted into the recipient sac under ultrasound guidance either directly using reusable trocars with pyramidal tips (Karl Storz) or by the ‘Seldinger’ technique for vascular access. The site of entry on the maternal abdomen is crucial when performing the procedure. Although optimal positioning is sometimes limited by the location of placenta, a site not covered by the placenta that maximizes the chance of visualizing the vascular equator of the placenta should be chosen whenever possible. The virtual vascular equator of the placenta can be envisioned based on the two cord insertion sites and the body axis of the donor.17
Figure 1.
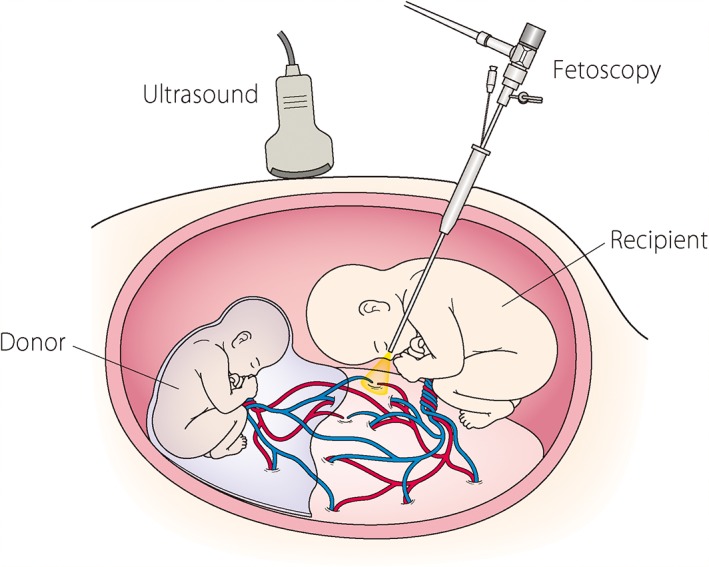
A schematic representation of fetoscopic laser photocoagulation for twin–twin transfusion syndrome. A fetoscope is percutaneously inserted into the recipient sac through a cannula.
Most operators use a 2‐mm fetoscope with a 3‐mm sheath (Karl Storz). Some operators use a 3.5‐mm diagnostic and a 3‐mm operating fetoscope (Richard Wolf). A 1.3‐mm fetoscope is also used for cases younger than 20 weeks of gestation. A rigid fetoscope with a straight sheath is used for a posterior placenta, and a semi‐rigid fetoscope with a curved sheath is used for an anterior placenta. A 600‐μm laser fiber is passed through the operative channel of the sheath of the fetoscope, and a neodymium: yttrium–aluminum–garnet (Nd:YAG) laser or diode (semi‐conductor) laser is used. Although a diode laser is cheaper, an Nd:YAG laser is more commonly used.
All communicating vessels between the twins on the chorionic plate of the placenta (including arteriovenous [AV], arterioarterial [AA] and venovenous [VV] anastomoses) are coagulated using an output of 20–60 watts with a nontouch technique. The goal of surgery is to ablate all intertwin anastomoses.
Coagulation methods
Advances in the coagulation methods have been made. Initially, all vessels crossing the dividing membrane were coagulated (nonselective coagulation).6 While this method interrupts vascular anastomoses between the twins, many vessels that are not involved in anastomoses are also coagulated. Unnecessarily coagulated vessels result in a reduced vascular territory, which leads to a higher risk of fetal death. To overcome this problem, only communicating vessels between the twins are coagulated (selective coagulation), a process that was first described by Quintero et al.18 These authors reported a sequential selective coagulation technique, in which the order of coagulation was determined by the type of anastomoses in order to reduce donor hypotension.19 A modified sequential selective coagulation technique was proposed by Nakata et al., involving the reversal of the order of coagulation of AA or VV anastomoses in the sequential coagulation.20 Coagulation of AV anastomoses follows that of AA and VV anastomoses. The ideal order of coagulation is still being debated. For selective coagulation, all vascular anastomoses must be identified. However, it is sometimes difficult to observe all of the vascular equator, in which communicating vessels are found, due to its location under the donor body. Thus, most procedures are performed as a combination of selective and nonselective coagulation.17
The recent modification of coagulation referred to as the ‘Solomon technique’ that coagulates the entire vascular equator has been reported.21, 22, 23 This technique was developed to reduce residual anastomoses that cause recurrence of TTTS or twin anemia–polycythemia sequence (TAPS).24 It was shown that the ‘Solomon technique’ was associated with a reduction in TAPS (3% vs 16% for the standard treatment) and recurrence of TTTS (1% vs 7%) by an open‐label randomized controlled trial.25 The ‘Solomon technique’ involves initially completing coagulation of all visible anastomoses and then performing coagulation to connect the anastomoses’ ablation sites from one edge of the placenta to the other (Fig. 2). This method enables MC placenta to be dichorionized by coagulating placental vessels and the surface of placenta. Although the occurrence of TAPS following FLP using selective coagulation was found to be low in our study (2.6%),26 we expect it to be further reduced using the ‘Solomon technique’.
Figure 2.
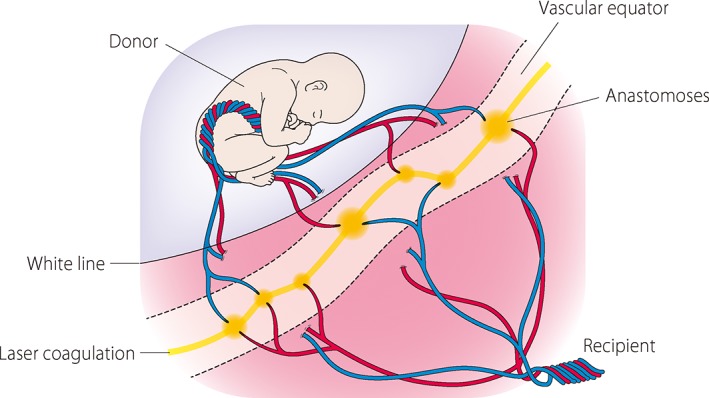
A schematic representation of the ‘Solomon technique’. Initially, all visible anastomoses are coagulated, and then coagulation is performed to connect the anastomoses’ ablation sites from one edge of the placenta to the other.
Survival outcomes
We reported our initial 181 consecutive cases of TTTS subjected to FLP at four centers in Japan between 2002 and 2006.27 The mean gestational ages at FLP and delivery were 21.2 and 32.9 weeks, respectively. The survivals of both twins and at least one twin at 6 months of age were 61.9% and 90.1%, respectively, and those in 148 cases between 2003 and 2009 at our center were 71.6% and 93.2%, respectively.28 A systematic review reported that the perinatal survival following FLP improved significantly with the evolution of the coagulation technique such as selective coagulation and the ‘Solomon technique’ and with a learning curve effect after the introduction of FLP in 1990.29 The mean survival of both twins increased from 35% to 65% and that for at least one twin from 70% to 88% over the past 25 years in a total of 34 studies including 3868 cases. The mean gestational age at delivery remained stable over the years at 32 weeks of gestation. The largest single‐center experience for FLP in TTTS with 1020 cases was reported.30 The survival of both twins increased from 50% in the first 200 cases to 69.5% in the latter 220 cases. The survival of at least one twin increased from 80.5% in the first 200 cases to 91.8% in the latter 220 cases. Thus, with the recent significant improvement of survival rates, survival rates of 70% for both twins and more than 90% for at least one twin can be expected after FLP.
Neurological outcomes
Despite an improved survival rate, TTTS treated with FLP is still associated with neurological abnormalities, including severe cerebral injury and neurodevelopment impairments. Severe cerebral injury such as intraventricular hemorrhaging, cystic periventricular leukomalacia, porencephaly and ventriculomegaly can be detected by ultrasound and/or magnetic resonance imaging in the short‐term follow‐up. However, neurodevelopment impairments, such as cerebral palsy, severe development delay, blindness and deafness, require a long‐term follow‐up to be assessed. The incidence of severe cerebral injury in the recent cohort ranged from 2.9% to 6%.27, 31, 32 Two systematic reviews with pooled studies revealed the incidence of neurodevelopment impairment to be 11.1% (140/1255) and 13.3% (83/624).33, 34 Gray et al. reported that the incidence of neurodevelopment impairment as assessed by the Griffiths scale at 2 years of age corrected for prematurity was 12.4% (14/113).35 Klink et al. reported that the incidence of neurodevelopment impairment assessed by the Bayley scale at 2 years of age corrected for prematurity was 12.4% (38/307).31 A recent single‐center study using the Ages and Stages Questionnaires showed that the incidence of neurodevelopment impairment between 2 and 5 years of age was 13.5% (17/126).32 Therefore, the risks of neurodevelopment impairment following FLP are assumed to range from 11% to 14%. The incidence of neurodevelopment impairment assessed by the Bayley scale at 2 years of age corrected for prematurity decreased from 18% (28/152; 2000–2005) to 6% (10/155; 2008–2010) over time.31 It is very important to confirm the improvements in the long‐term neurodevelopment outcomes with advances in the technique for FLP.
Complications
Preterm premature rupture of membranes (pPROM), which leads to preterm labor, is a common complication after FLP.17 The incidence of pPROM within 7 and 28 days after FLP was 3.9–6% and 7.7–9%, respectively.10, 27 Chorioamniotic membrane separation, an iatrogenic complication, occurs in approximately 20% of patients following FLP and is associated with pPROM before 28 weeks of gestation.28, 36 No effective therapies to treat and prevent iatrogenic pPROM have yet been developed.
Maternal complications have also been reported in 10.7% of 150 FLP cases, with 6.0% classified as major and 4.7% as mild.37 Major maternal complications included placental abruption, accounting for the majority, as well as amniotic fluid embolism and Mirror syndrome.37 We experienced one case of placental abruption and two cases of Mirror syndrome in 181 FLP cases.27 Mild maternal complications able to be managed conservatively included intraperitoneal amniotic fluid leakage and bleeding from the uterine wall. Attention for maternal complications should be paid in the management of pregnancy and labor of MC twins following FLP.
Conventional criteria
FLP is offered as the first‐line treatment option for TTTS at 16–26 weeks of gestation, which is thought to be the conventional criterion (Table 1).10, 11, 27 There has been some debate recently about the use of FLP to manage TTTS stage 1. A number of TTTS stage 1 cases do not progress or even regress. It was suggested that conservative management of TTTS stage 1 was a reasonable option,38 based on the overall survival rates of 86% in cases of conservative management, 77% after amnioreduction and 85% after FLP in a literature review. The outcome of FLP is key to its application for TTTS stage 1. A recent systematic review of the outcomes of stage 1 TTTS revealed that the survival rates of both twins and at least one twin with expectant management were 70% and 87%, respectively, while those after amnioreduction were 67% and 86%, respectively, and those after FLP 54% and 81%, respectively.39 However, considering recent significant improvements in the survival rates of FLP, a 70% survival rate for both twins and more than 90% survival rate for at least one twin can be expected for all stages of TTTS treated by FLP. These favorable rates therefore make the conservative management of stage 1 TTTS less attractive as a therapeutic option.
Table 1.
Our criteria for performing fetoscopic laser photocoagulation
| Conventional criteria |
| Twin–twin transfusion syndrome at 16–26 weeks |
| • Monochorionic twin pregnancy |
| • Oligohydramnios with an MVP ≤ 2.0 cm in the donor |
| • Polyhydramnios with an MVP ≥ 8.0 cm in the recipient |
| • Gestational age between 16 + 0 and 25 + 6 weeks of gestation |
| Nonconventional criteria |
| Triplets |
| • Dichorionic triamniotic triplets or monochorionic triamniotic triplets |
| • Feto‐fetal transfusion syndrome (MVP ≤ 2.0 cm in the donor and ≥8.0 cm in the recipient) |
| • Gestational age between 16 + 0 and 25 + 6 weeks of gestation |
| Twin–twin transfusion syndrome after 26 weeks |
| • Monochorionic twin pregnancy |
| • Oligohydramnios with an MVP ≤ 2.0 cm in the donor |
| • Polyhydramnios with an MVP ≥ 10.0 cm in the recipient |
| • Gestational age between 26 + 0 and 27 + 6 weeks of gestation |
| Selective intrauterine growth restriction with oligohydramnios |
| • Monochorionic twin pregnancy |
| • Estimated fetal weight ≤ −1.5 SD or intertwin estimated fetal weight discordance ≥25% |
| • Absent or reverse end‐diastolic velocity in the umbilical artery in the smaller twin |
| • Oligohydramnios with an MVP ≤ 2.0 cm in the smaller twin |
| • Gestational age between 16 + 0 and 25 + 6 weeks of gestation |
MVP, maximum vertical pocket; SD, standard deviation.
The North American Fetal Therapy Network has shown interesting and important results.40 Stage 1 TTTS was associated with considerable fetal mortality. Although spontaneous recovery from TTTS was observed in some cases, TTTS progressed in the majority of expectantly managed cases, leading to a worse prognosis. Both amnioreduction and FLP decreased the risk of no survivors, and FLP was able to substantially prevent a poor outcome. FLP is the only therapy that directly halts the pathologic process. Therefore, it is reasonable to use FLP for stage 1 TTTS unless a randomized clinical trial reveals marked disadvantages associated with FLP.
Triplets
Triplet gestations, including dichorionic triamniotic (DT) triplets and monochorionic triamniotic (MT) triplets, are associated with high risks for feto‐fetal transfusion syndrome (FFTS), which leads to perinatal death and neurological impairments.41 FFTS in triplets corresponds to TTTS in twins. FLP is thought to be useful for treating FFTS in triplets. Several case series on triplet gestations treated by FLP have been described.42, 43 Sepulveda et al. reported 10 cases (seven of DT and three of MT) in which the overall perinatal survival in DT was 66.7% while that in MT was 22.2%.42 We reported 16 cases (nine of DT and seven of MT).43 The median gestational ages at surgery and at delivery were 21 and 31 weeks, respectively. The overall perinatal survival in DT was 74% while that of MT was 95%. All 16 cases resulted in at least one survival. FLP is a valuable treatment option for FFTS in both DT and MT triplets.
After 26 weeks of gestation
The gestational age for the treatment of TTTS was limited to 16–26 weeks in the first randomized trial for FLP.10 This gestational age limit for FLP has widely been adopted; however, cases of TTTS after 26 weeks can prove difficult to treat. The expected burdens associated with performing FLP after 26 weeks include the amniotic fluid potentially becoming more turbid, thereby hampering visualization of the placental anastomoses, and larger placental vessels potentially becoming more difficult to ablate. Valsky et al. compared the outcomes of 28 TTTS cases treated with FLP after 26 weeks with 324 cases treated between 16 and 26 weeks of gestation.44 Baud et al. investigated the outcomes of 18 TTTS cases treated with FLP after 26 weeks with 283 cases treated between 17 and 26 weeks of gestation.45 Both studies showed that FLP for TTTS after 26 weeks of gestation was associated with similar outcomes to cases treated before 26 weeks.44, 45 We performed a small prospective study investigating all cases of TTTS with an MVP ≥10.0 cm in the recipient at 26–28 weeks of gestation during 2012 and 2013.46 Six cases were enrolled and underwent FLP. FLP was completely achieved in all six cases and resulted in the survival of both twins. FLP may still be a safe therapeutic option for TTTS after 26 weeks, especially at 26–28 weeks of gestation.
Amniotic fluid discordance and selective intrauterine growth restriction
With the increased awareness of TTTS, greater attention is now being paid to MC twin pregnancies complicated with amniotic fluid discordance (AFD) and/or selective intrauterine growth restriction (sIUGR). AFD is diagnosed based on the difference in the amniotic fluid volume of twins, which is thought to be due to discordance in the placental blood flow between the twins. sIUGR is diagnosed based on the difference in the fetal body weight of twins, which is thought to be due to discordance in the shared placental area. Although the concept of AFD differs from that of sIUGR, AFD can overlap with sIUGR. AFD with sIUGR and absent or reverse end‐diastolic velocity (AREDV) in the umbilical artery (UA) in one fetus is associated with an extremely high risk for adverse pregnancy outcomes.47 However, the clinical management of AFD or sIUGR without signs of TTTS is still controversial, and the application of FLP for AFD or sIUGR is challenging. We performed FLP on 11 cases of AFD bordering on TTTS with AREDV in the UA in one fetus, which represents a high‐risk condition for adverse pregnancy outcomes.48 The overall and both‐twin survival rates were 63.6% and 27.7%, respectively. In our previous study, under expectant management, the overall and both‐twin survival rates were 57.9% and 42.1%, respectively.49 Our preliminary study shows that FLP does not seem to be a promising treatment option for AFD bordering on TTTS.
Several reports have described the application of FLP for sIUGR with AREDV in the UA. The survival rates of IUGR twins following FLP were 45.5% (5/11), 25% (4/16) and 30.4% (7/23) in the relevant reports, while those of larger twins was 63.6% (7/11), 93.7% (15/16) and 73.9% (17/23), respectively.50, 51, 52 We showed the natural history and prognostic factors of sIUGR. Of 101 sIUGR twins, 70 (69.3%) IUGR twins and 82 (81.2%) larger twins survived.53 Considering our survival rates of sIUGR twins treated with expectant management, the outcomes of FLP for sIUGR do not appear to be promising. We also showed that both AREDV in UA and severe oligohydramnios were important indicators for the mortality of sIUGR. In 101 sIUGR twins, 11 cases had both AREDV in UA and severe oligohydramnios, and only one IUGR twin (9%) with neurological abnormalities and four larger twins (36%) survived.54 We performed FLP in 10 cases of sIUGR with both AREDV in UA and severe oligohydramnios. 55 Three IUGR twins (30%) and 10 larger twins (100%) were alive without neurological abnormalities. Recently, we achieved similar results with more than 50 sIUGR cases (Ishii K et al., 2017, unpublished data). FLP for sIUGR may be beneficial when accompanied by both AREDV in UA and severe oligohydramnios of IUGR twins. Oligohydramnios in the IUGR twin may be a key indicator to perform FLP for sIUGR. The FLP criteria for nonconventional applications, including triplets, TTTS after 26 weeks and sIUGR, are shown in Table 1.
Status in Japan
The milestones of FLP worldwide and in Japan are shown in Table 2. In Japan, 10 years after the first case was performed via laparotomy,56 an FLP program was started in 2002 in Hamamatsu and several months later in our center. Several centers followed in the next several years. These centers formed the Japan Fetoscopy Group, which succeeded the Japan Fetal Therapy Group, a research group for the treatment of unborn patients in Japan. 57 Our findings resulted in FLP being recognized as the first‐line treatment option for TTTS in Japan, with the cost of the FLP procedure being covered by Japan National Health Insurance from 2012. FLP is the first fetal treatment to be covered by Japan National Health Insurance, implying that fetuses are now recognized as patients by the government. The annual number of FLP procedures performed in Japan is shown in Figure 3. The numbers of procedures were collected from all institutions that performed FLP as members of the Japan Fetal Therapy Group. 57 Only five institutions performed FLP in 2005, with the number increasing to 10 in 2016. The number of FLP procedures has been increasing annually and exceeded 200 cases per year, with the total reaching 1660 in 2016. The birth number in Japan in 2016 was about one million. Two cases of FLP per 10 000 births were performed in 2016.
Table 2.
Milestones of fetoscopic laser photocoagulation worldwide and in Japan
| Year | World | Japan |
|---|---|---|
| 1990 | De Lia; three cases via laparotomy5 | |
| 1992 | Natori; one case via laparotomy56 | |
| 1995 | Ville; 45 cases via the percutaneous approach6 | |
| 1998 | Ville; 132 cases7 | |
| Quintero; selective coagulation18 | ||
| 1999 | Quintero; TTTS staging12 | |
| 2002 | Start FLP program | |
| 2004 | Senat; a randomized trial for FLP versus amnioreduction10 | |
| 2010 | Sago; 181 cases27 | |
| 2012 | Coverage by National Health Insurance | |
| 2014 | Slaghekke; a randomized trial for Solomon technique25 |
FLP, fetoscopic laser photocoagulation; TTTS, twin–twin transfusion syndrome.
Figure 3.
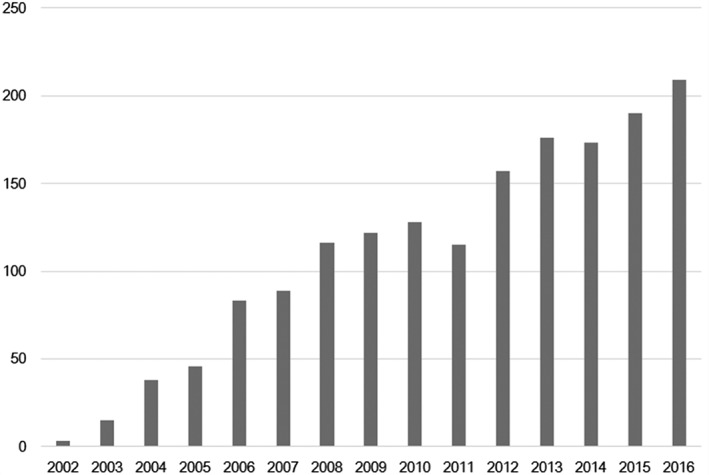
The number of fetoscopic laser photocoagulation procedures performed annually in Japan.
Our center, the National Center for Child Health and Development, is the most active center for fetal diagnoses and fetal therapy in Japan. A total of 794 women received fetal treatment at our center in 2002–2016. FLP was used to treat 561 TTTS patients (70.1% of all fetal therapy cases). Thoracoamniotic shunting (TAS) was performed in 70 cases of severe fetal hydrothorax (8.8% of all fetal therapy cases). Radiofrequency ablation (RFA) was performed to discontinue the blood flow to an acardiac twin and to save the normal‐pump twin in 51 cases of twin‐reversed arterial perfusion sequence (6.4% of all fetal therapy cases). We conducted a safety and feasibility study of fetoscopic endoluminal tracheal occlusion in the treatment of severe congenital diaphragmatic hernia in 11 cases (1.4% of all fetal therapy cases). The survival rate of TAS in fetal hydrothorax with hydrops was around 63%. 58 The overall survival of the normal‐pump twin following RFA was 85%.59 FLP is the most commonly performed and successful fetal intervention at present.
Conclusion
More than 25 years have passed since the first attempt at FLP, and 15 years have passed since the introduction of the FLP program in Japan. Great progress has been made over time, with a number of advances made in techniques for FLP for the treatment of TTTS. FLP is the optimal treatment option for TTTS at 16–26 weeks of gestation. The outcomes following FLP have been dramatically improved, with survival rates of more than 90% for at least one twin and 70% for both twins. However, there is still an 11–14% risk of long‐term neurodevelopment impairment. FLP may be a therapeutic option for FFTS in triplets and TTTS after 26 weeks as well as for sIUGR associated with AREDV in UA and oligohydramnios. FLP is the most commonly performed and successful fetal intervention to date. Focus in the near future should be placed on improving the neurodevelopmental outcomes.
Disclosure
None declared.
Acknowledgments
This work was supported by grants from The Grants of National Center for Child Health and Development, Tokyo, Japan (Grant number: 27‐3, 29‐13). We thank Dr Takeshi Murakoshi (Seirei Hamamatsu General Hospital), Dr Masahiko Nakata (Toho University), Dr Jun Murotsuki (Miyagi Children's Hospital) and Dr Yuichiro Takahashi (Nagara Medical Center) for their long‐term cooperation in the research and practice of FLP as members of the Japan Fetoscopy Group, which succeeded the Japan Fetal Therapy Group.
The copyright line for this article was changed on March 2, 2018 after original online publication.
References
- 1. Mahony BS, Petty CN, Nyberg DA, Luthy DA, Hickok DE, Hirsch JH. The ‘stuck twin’ phenomenon: Ultrasonographic findings, pregnancy outcome, and management with serial amniocenteses. Am J Obstet Gynecol 1990; 163: 1513–1522. [DOI] [PubMed] [Google Scholar]
- 2. Saunders NJ, Snijders RJ, Nicolaides KH. Therapeutic amniocentesis in twin‐twin transfusion syndrome appearing in the second trimester of pregnancy. Am J Obstet Gynecol 1992; 166: 820–824. [DOI] [PubMed] [Google Scholar]
- 3. Diehl W, Hecher K, Zikulnig L, Vetter M, Hackelöer BJ. Placental vascular anastomoses visualized during fetoscopic laser surgery in severe mid‐trimester twin‐twin transfusion syndrome. Placenta 2001; 22: 876–881. [DOI] [PubMed] [Google Scholar]
- 4. Bermúdez C, Becerra CH, Bornick PW, Allen MH, Arroyo J, Quintero RA. Placental types and twin‐twin transfusion syndrome. Am J Obstet Gynecol 2002; 187: 489–494. [DOI] [PubMed] [Google Scholar]
- 5. De Lia JE, Cruikshank DP, Keye WR. Fetoscopic neodymium: YAG laser occlusion of placental vessels in severe twin‐twin transfusion syndrome. Obstet Gynecol 1990; 75: 1046–1053. [PubMed] [Google Scholar]
- 6. Ville Y, Hyett J, Hecher K, Nicolaides K. Preliminary experience with endoscopic laser surgery for severe twin‐twin transfusion syndrome. N Engl J Med 1995; 332: 224–227. [DOI] [PubMed] [Google Scholar]
- 7. Ville Y, Hecher K, Gagnon A, Sebire N, Hyett J, Nicolaides K. Endoscopic laser coagulation in the management of severe twin‐to‐twin transfusion syndrome. Br J Obstet Gynaecol 1998; 105: 446–453. [DOI] [PubMed] [Google Scholar]
- 8. Hecher K, Plath H, Bregenzer T, Hansmann M, Hackelöer BJ. Endoscopic laser surgery versus serial amniocenteses in the treatment of severe twin‐twin transfusion syndrome. Am J Obstet Gynecol 1999; 180: 717–724. [DOI] [PubMed] [Google Scholar]
- 9. Quintero RA, Dickinson JE, Morales WJ et al Stage‐based treatment of twin‐twin transfusion syndrome. Am J Obstet Gynecol 2003; 188: 1333–1340. [DOI] [PubMed] [Google Scholar]
- 10. Senat MV, Deprest J, Boulvain M, Paupe A, Winer N, Ville Y. Endoscopic laser surgery versus serial amnioreduction for severe twin‐to‐twin transfusion syndrome. N Engl J Med 2004; 351: 136–144. [DOI] [PubMed] [Google Scholar]
- 11. Rossi AC, D'Addario V. Laser therapy and serial amnioreduction as treatment for twin‐twin transfusion syndrome: A meta‐analysis and review of literature. Am J Obstet Gynecol 2008; 198: 147–152. [DOI] [PubMed] [Google Scholar]
- 12. Quintero RA, Morales WJ, Allen MH, Bornick PW, Johnson PK, Kruger RM. Staging of twin‐twin transfusion syndrome. J Perinatol 1999; 19: 550–555. [DOI] [PubMed] [Google Scholar]
- 13. Murakoshi T, Ishii K, Nakata M et al Validation of the Quintero's stage III sub‐classification for twin‐twin transfusion syndrome with visible or non‐visible donor bladder: Insight into arterio‐arterial anastomoses and umbilical arterial Doppler. Ultrasound Obstet Gynecol 2008; 32: 813–818. [DOI] [PubMed] [Google Scholar]
- 14. Galea P, Jain V, Fisk NM. Insights into the pathophysiology of twin‐twin transfusion syndrome. Prenat Diagn 2005; 25: 777–785. [DOI] [PubMed] [Google Scholar]
- 15. Rychik J, Tian Z, Bebbington M et al The twin‐twin transfusion syndrome: Spectrum of cardiovascular abnormality and development of a cardiovascular score to assess severity of disease. Am J Obstet Gynecol 2007; 197: 392 e1–392 e8. [DOI] [PubMed] [Google Scholar]
- 16. Shah AD, Border WL, Crombleholme TM, Michelfelder EC. Initial fetal cardiovascular profile score predicts recipient twin outcome in twin‐twin transfusion syndrome. J Am Soc Echocardiogr 2008; 21: 1105–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chalouhi GE, Essaoui M, Stirnemann J et al Laser therapy for twin‐to‐twin transfusion syndrome (TTTS). Prenat Diagn 2011; 31: 637–646. [DOI] [PubMed] [Google Scholar]
- 18. Quintero RA, Morales WJ, Mendoza G et al Selective photocoagulation of placental vessels in twin‐twin transfusion syndrome: Evolution of a surgical technique. Obstet Gynecol Surv 1998; 53: S97–S103. [DOI] [PubMed] [Google Scholar]
- 19. Quintero RA, Ishii K, Chmait RH, Bornick PW, Allen MH, Kontopoulos EV. Sequential selective laser photocoagulation of communicating vessels in twin‐twin transfusion syndrome. J Matern Fetal Neonatal Med 2007; 20: 763–768. [DOI] [PubMed] [Google Scholar]
- 20. Nakata M, Murakoshi T, Sago H et al Modified sequential laser photocoagulation of placental communicating vessels for twin‐twin transfusion syndrome to prevent fetal demise of the donor twin. J Obstet Gynaecol Res 2009; 35: 640–647. [DOI] [PubMed] [Google Scholar]
- 21. Baschat AA, Barber J, Pedersen N, Turan OM, Harman CR. Outcome after fetoscopic selective laser ablation of placental anastomoses vs equatorial laser dichorionization for the treatment of twin‐to‐twin transfusion syndrome. Am J Obstet Gynecol 2013; 209: 234 e1–234 e8. [DOI] [PubMed] [Google Scholar]
- 22. Ruano R, Rodo C, Peiro JL et al Fetoscopic laser ablation of placental anastomoses in twin‐twin transfusion syndrome using ‘Solomon technique’. Ultrasound Obstet Gynecol 2013; 42: 434–439. [DOI] [PubMed] [Google Scholar]
- 23. Slaghekke F, Lewi L, Middeldorp JM et al Residual anastomoses in twin‐twin transfusion syndrome after laser: The Solomon randomized trial. Am J Obstet Gynecol 2014; 211: 285.e1–285.e7. [DOI] [PubMed] [Google Scholar]
- 24. Slaghekke F, Kist WJ, Oepkes D et al Twin anemia‐polycythemia sequence: Diagnostic criteria, classification, perinatal management and outcome. Fetal Diagn Ther 2010; 27: 181–190. [DOI] [PubMed] [Google Scholar]
- 25. Slaghekke F, Lopriore E, Lewi L et al Fetoscopic laser coagulation of the vascular equator versus selective coagulation for twin‐to‐twin transfusion syndrome: An open‐label randomized controlled trial. Lancet 2014; 383: 2144–2151. [DOI] [PubMed] [Google Scholar]
- 26. Taniguchi K, Sumie M, Sugibayashi R, Wada S, Matsuoka K, Sago H. Twin anemia‐polycythemia sequence after laser surgery for twin‐twin transfusion syndrome and maternal morbidity. Fetal Diagn Ther 2015; 37: 148–153. [DOI] [PubMed] [Google Scholar]
- 27. Sago H, Hayashi S, Saito M et al The outcome and prognostic factors of twin‐twin transfusion syndrome following fetoscopic laser surgery. Prenat Diagn 2010; 30: 1185–1191. [DOI] [PubMed] [Google Scholar]
- 28. Egawa M, Hayashi S, Yang L, Sakamoto N, Sago H. Chorioamniotic membrane separation after fetoscopic laser surgery for twin‐twin transfusion syndrome. Prenat Diagn 2013; 33: 89–94. [DOI] [PubMed] [Google Scholar]
- 29. Akkermans J, Peeters SH, Klumper FJ, Lopriore E, Middeldorp JM, Oepkes D. Twenty‐five years of fetoscopic laser coagulation in twin‐twin transfusion syndrome: A systematic review. Fetal Diagn Ther 2015; 38: 241–253. [DOI] [PubMed] [Google Scholar]
- 30. Diehl W, Diemert A, Grasso D, Sehner S, Wegscheider K, Hecher K. Fetoscopic laser coagulation in 1020 pregnancies with twin‐to‐twin transfusion syndrome demonstrates improvement of double survival rates. Ultrasound Obstet Gynecol 2017; 50: 728–735. [DOI] [PubMed] [Google Scholar]
- 31. van Klink JM, Koopman HM, van Zwet EW et al Improvement in neurodevelopmental outcome in survivors of twin‐twin transfusion syndrome treated with laser surgery. Am J Obstet Gynecol 2014; 210: 540 e1–540 e7. [DOI] [PubMed] [Google Scholar]
- 32. Sananès N, Gabriele V, Weingertner AS et al Evaluation of long‐term neurodevelopment in twin‐twin transfusion syndrome after laser therapy. Prenat Diagn 2016; 36: 1139–1145. [DOI] [PubMed] [Google Scholar]
- 33. Rossi AC, Vanderbilt D, Chmait RH. Neurodevelopmental outcomes after laser therapy for twin‐twin transfusion syndrome: A systematic review and meta‐analysis. Obstet Gynecol 2011; 118: 1145–1150. [DOI] [PubMed] [Google Scholar]
- 34. van Klink JM, Koopman HM, Oepkes D, Walther FJ, Lopriore E. Long‐term neurodevelopmental outcome in monochorionic twins after fetal therapy. Early Hum Dev 2011; 87: 601–606. [DOI] [PubMed] [Google Scholar]
- 35. Gray PH, Poulsen L, Gilshenan K et al Neurodevelopmental outcome and risk factors for disability for twin‐twin transfusion syndrome treated with laser surgery. Am J Obstet Gynecol 2011; 204: 159.e1–159.e6. [DOI] [PubMed] [Google Scholar]
- 36. Papanna R, Mann LK, Johnson A, Sangi‐Haghpeykar H, Moise KJ Jr. Chorioamnion separation as a risk for preterm premature rupture of membranes after laser therapy for twin‐twin transfusion syndrome. Obstet Gynecol 2010; 115: 771–776. [DOI] [PubMed] [Google Scholar]
- 37. Rustico MA, Lanna MM, Faiola S et al Fetal and maternal complications after selective fetoscopic laser surgery for twin‐to‐twin transfusion syndrome: A single‐center experience. Fetal Diagn Ther 2012; 31: 170–178. [DOI] [PubMed] [Google Scholar]
- 38. Rossi AC, D'Addario V. Survival outcomes of twin‐twin transfusion syndrome stage I: A systematic review of literature. Am J Perinatol 2013; 30: 5–10. [DOI] [PubMed] [Google Scholar]
- 39. Khalil A, Cooper E, Townsend R, Thilaganathan B. Evolution of stage 1 twin‐to‐twin transfusion syndrome (TTTS): Systematic review and meta‐analysis. Twin Res Hum Genet 2016; 19: 207–216. [DOI] [PubMed] [Google Scholar]
- 40. Emery SP, Hasley SK, Catov JM et al North American fetal therapy network. North American fetal therapy network: Intervention vs expectant management for stage I twin‐twin transfusion syndrome. Am J Obstet Gynecol 2016; 215: 346 e1–346 e7. [DOI] [PubMed] [Google Scholar]
- 41. Sato Y, Ishii K, Yokouchi T et al Incidences of feto‐fetal transfusion syndrome and perinatal outcomes in triplet gestations with monochorionic placentation. Fetal Diagn Ther 2016; 40: 181–186. [DOI] [PubMed] [Google Scholar]
- 42. Sepulveda W, Surerus E, Vandecruys H, Nicolaides KH. Fetofetal transfusion syndrome in triplet pregnancies: Outcome after endoscopic laser surgery. Am J Obstet Gynecol 2005; 192: 161–164. [DOI] [PubMed] [Google Scholar]
- 43. Ishii K, Nakata M, Wada S, Hayashi S, Murakoshi T, Sago H. Perinatal outcome after laser surgery for triplet gestations with feto‐fetal transfusion syndrome. Prenat Diagn 2014; 34: 734–738. [DOI] [PubMed] [Google Scholar]
- 44. Valsky DV, Eixarch E, Martinez‐Crespo JM et al Fetoscopic laser surgery for twin‐to‐twin transfusion syndrome after 26 weeks of gestation. Fetal Diagn Ther 2012; 31: 30–34. [DOI] [PubMed] [Google Scholar]
- 45. Baud D, Windrim R, Keunen J et al Fetoscopic laser therapy for twin‐twin transfusion syndrome before 17 and after 26 weeks' gestation. Am J Obstet Gynecol 2013; 208: 197 e1–197 e7. [DOI] [PubMed] [Google Scholar]
- 46. Nakata M, Ishii K, Sumie M et al A prospective pilot study of fetoscopic laser surgery for twin‐to‐twin transfusion syndrome between 26 and 27 weeks of gestation. Taiwan J Obstet Gynecol 2016; 55: 512–514. [DOI] [PubMed] [Google Scholar]
- 47. Huber A, Diehl W, Zikulnig L, Bregenzer T, Hackelöer BJ, Hecher K. Perinatal outcome in monochorionic twin pregnancies complicated by amniotic fluid discordance without severe twin‐twin transfusion syndrome. Ultrasound Obstet Gynecol 2006; 27: 48–52. [DOI] [PubMed] [Google Scholar]
- 48. Ozawa K, Sugibayashi R, Wada S et al Fetoscopic laser photocoagulation for amniotic fluid discordance bordering on twin‐twin transfusion syndrome: Feasibility, perinatal and long‐term outcomes. J Obstet Gynaecol Res 2017; 43: 1256–1262. [DOI] [PubMed] [Google Scholar]
- 49. Hayashi S, Anami A, Ishii K et al Outcome of monochorionic twin pregnancies with moderate amniotic fluid discordance adjoining twin‐twin transfusion syndrome. Prenat Diagn 2016; 36: 170–176. [DOI] [PubMed] [Google Scholar]
- 50. Quintero RA, Bornick PW, Morales WJ, Allen MH. Selective photocoagulation of communicating vessels in the treatment of monochorionic twins with selective growth retardation. Am J Obstet Gynecol 2001; 185: 689–696. [DOI] [PubMed] [Google Scholar]
- 51. Gratacos E, Antolin E, Lewi L et al Monochorionic twins with selective intrauterine growth restriction and intermittent absent or reversed end‐diastolic flow (TypeIII): Feasibility and perinatal outcome of fetoscopic placental laser coagulation. Ultrasound Obstet Gynecol 2008; 31: 669–675. [DOI] [PubMed] [Google Scholar]
- 52. Chalouhi GE, Marangoni MA, Quibel T et al Active management of selective intrauterine growth restriction with abnormal Doppler in monochorionic diaminiotic twin pregnancies diagnosed in the second trimester of pregnancy. Prenat Diagn 2013; 33: 109–115. [DOI] [PubMed] [Google Scholar]
- 53. Ishii K, Murakoshi T, Hayashi S et al Ultrasound predictors of mortality in monochorionic twins with selective intrauterine growth restriction. Ultrasound Obstet Gynecol 2011; 37: 22–26. [DOI] [PubMed] [Google Scholar]
- 54. Ishii K, Murakoshi T, Sago H. Adverse outcome in monochorionic twins with selective intrauterine fetal growth restriction in the presence of abnormal umbilical artery Doppler and severe oligohydramnios. J Obstet Gynaecol Res 2012; 38: 1271. [DOI] [PubMed] [Google Scholar]
- 55. Ishii K, Nakata M, Wada S, Murakoshi T, Sago H. Feasibility and preliminary outcomes of fetoscopic laser photocoagulation for monochorionic twin gestation with selective intrauterine growth restriction accompanied by severe oligohydramonios. J Obstet Gynaecol Res 2015; 41: 1732–1737. [DOI] [PubMed] [Google Scholar]
- 56. Natori M, Tanaka M, Kohno H et al A case of twin‐twin transfusion syndrome treated with placental vessel occlusion using fetoscopic Nd:YAG laser system. Nihon Sanka Fujinka Gakkai Zasshi 1992; 44: 117–120. (In Japanese.) [PubMed] [Google Scholar]
- 57. Japan Fetal Therapy Group . Fetal diagnosis, therapy and clinical research in Japan. [Cited 24 2017.] Available from URL: https://fetusjapan.jp
- 58. Wada S, Jwa SC, Yumoto Y et al The prognostic factors and outcomes of primary fetal hydrothorax with the effects of fetal intervention. Prenat Diagn 2017; 37: 184–192. [DOI] [PubMed] [Google Scholar]
- 59. Sugibayashi R, Ozawa K, Sumie M, Wada S, Ito Y, Sago H. Forty cases of twin reversed arterial perfusion sequence treated with radio frequency ablation using the multistep coagulation method: A single‐center experience. Prenat Diagn 2016; 36: 437–443. [DOI] [PubMed] [Google Scholar]


