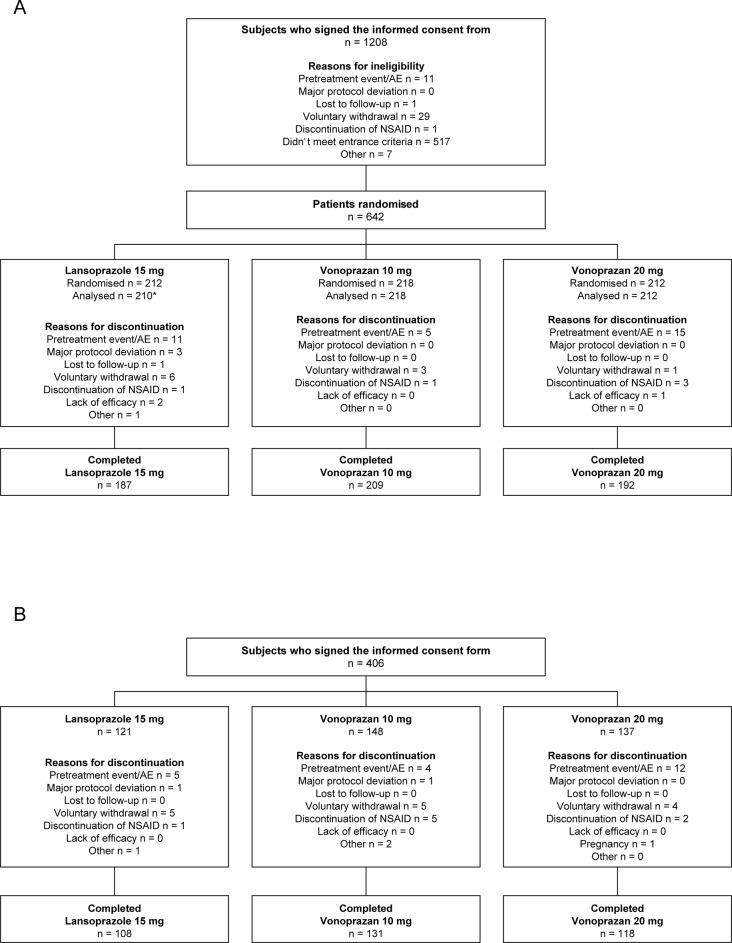
Figure 1.
Patient disposition in the double-blind (A) and extension (B) studies.(A) Phase 3, multicenter, randomised, double-blind, parallel-group, non-inferiority study conducted to evaluate the non-inferiority of vonoprazan to lansoprazole in preventing occurrence of secondary ulcers in patients with a history of endoscopically confirmed gastric or duodenal ulcer (peptic ulcer) who require long-term NSAID therapy. (B) Phase 3, multicenter, single-blind, parallel-group extension study to evaluate the safety and ulcer recurrence during long-term drug exposure; subjects who completed the non-inferiority study were eligible for enrolment. One patient did not receive study medication. One patient was excluded due to protocol violations.