Fig. 7.
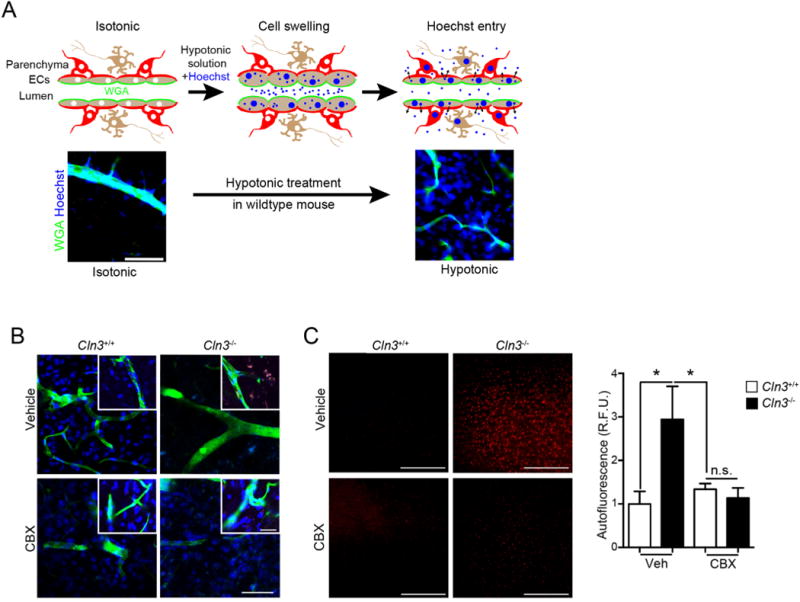
CBX corrects regulated volume defects and reduces Cln3−/− autofluorescent inclusions in vivo. A, (Top) Schematic representing Hoechst entry into the brain during hypotonic treatment. (Bottom) Cln3+/− mice exposed to isotonic or hypotonic treatments. Animals were anesthetized and wheat germ agglutinin (WGA) (green) infused intravascularly to label endothelial cells followed by infusion of Hoechst dye (blue) in a hypotonic solution, then saline infusion, followed by fixation, sectioning and imaging. The Hoechst signal outside the WGA-labeled vascular is indicative of regulated volume decrease and dye extravasation after hypotonicity exposure. Scale bar = 100 μm. B, Cln3+/+ or Cln3−/− mice were treated daily with vehicle or 20 mg/kg CBX for two weeks. After anesthetization, mice were perfused as in (A). Brain slices (50 μm) were imaged by confocal microscopy and show that CBX permits Hoechst dye extravasation in Cln3−/− mice brain. Images are representative of 5 mice per group performed on different days. Scale bar = 50 μm. C, Thin sections from fixed brains were imaged under low magnification in the red channel to detect autofluorescence. Scale bar = 200 μm. Cortical images (4 images/animal; 4 mice/group) were taken and fluorescence intensity averaged using ImageJ. Data are mean ± s.e.m., Kruska-Wallis test with Tukey’s multiple comparisons post-hoc, *, p < 0.05. Scale bar is 200 μm. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
