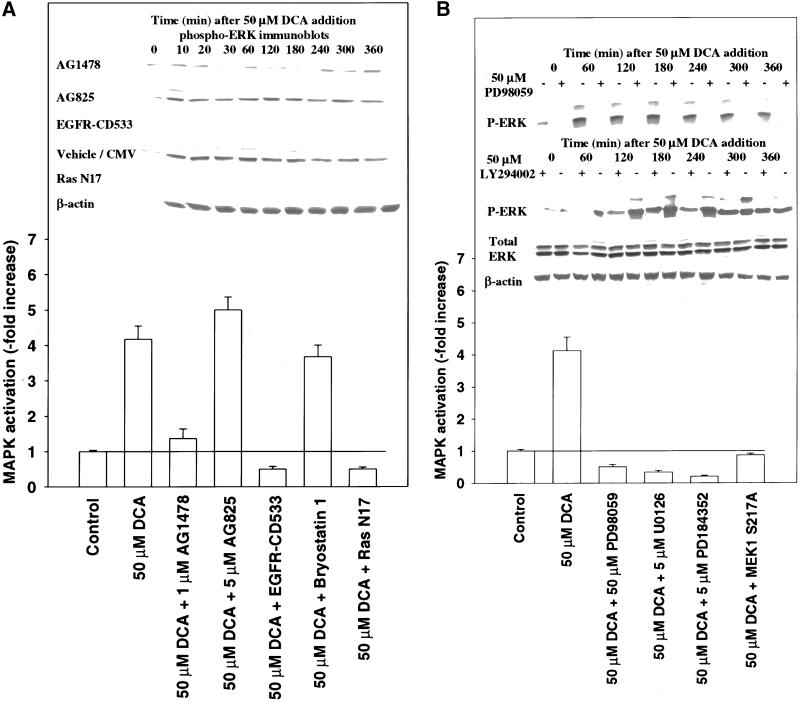
Figure 2.
DCA-induced activation of MAPK proceeds via the proto-oncogene Ras and not via classical PKC isoforms. (A) Cells were either pretreated with vehicle control (DMSO, VEH), bryostatin 1 (10 nM), or infected with either recombinant adenoviruses to express Ras N17, EGFR-CD533, or a null virus. Twenty-four hours after initial treatment/infection, cells were exposed to DCA (50 μM) and/or vehicle control (H2O, VEH), and 20 min after exposure, MAPK immunoprecipitated. Cells were lysed and portions (∼100 μg) from each plate well used to immunoprecipitate MAPK followed by immune-complex kinase assays as described in MATERIALS AND METHODS. (Inset) Cells were treated with 50 μM DCA in the presence or absence of either EGFR-CD533 or Ras N17 and MAPK activity determined >0–360 min by immunoblotting of cell lysates. Lysates were subjected to SDS-PAGE followed by immunoblotting versus antiphospho-MAPK antibody. A representative experiment is shown (n = 3). (B) DCA activates MAPK in hepatocytes, which is blocked by multiple small molecular weight chemical inhibitors of MEK1/2, dominant negative MEK1, and inhibitors of PI3 kinase. Cells were infected with either poly-l-lysine–conjugated adenoviruses to express either dominant negative MEK1 S217A or a null virus. Twenty-four hours after infection, cells were pretreated with either vehicle control (DMSO, VEH) or with PD98059 (50 μM), U0126 (5 μM), PD184352 (5 μM), wortmanin (5 μM), or LY294002 (50 μM). Cells were exposed to DCA (50 μM) and MAPK was activity determined 0–360 min after addition, via immunoblotting of cell lysates. Lysates were subjected to SDS-PAGE followed by immunoblotting versus antiphospho-MAPK antibody. A representative experiment is shown (n = 3).