Abstract
Background
Delirium significantly affects postoperative outcomes, but the incidence, risk factors and the long-term impact of delirium in lung transplant recipients have not been well studied.
Methods
We analyzed 155 lung transplant recipients enrolled in the Lung Transplant Outcomes Group (LTOG) cohort at a single center. We determined delirium incidence by structured chart review, identified risk factors for delirium, determined if plasma concentrations of two cerebral injury markers (neuron specific enolase [NSE] and glial fibrillary acidic protein [GFAP]) were associated with delirium, and determined the association of postoperative delirium with 1-year survival.
Results
Fifty-seven (36.8%) patients developed postoperative delirium. Independent risk factors for delirium included pre-transplant benzodiazepine prescription (relative risk [RR] 1.82; 95% confidence interval [CI]: 1.08, 3.07; p = 0.025), total ischemic time (RR 1.10 per 30 minute increase; 95% CI: 1.01, 1.21; p = 0.027), duration of time with intraoperative mean arterial pressure <60 mmHg (RR 1.07 per 15 minute increase; 95% CI: 1.00, 1.14; p = 0.041) and grade 3 primary graft dysfunction (RR 2.13; 95% CI: 1.27, 3.58; p = 0.004). Ninety-one (58.7%) patients had available plasma 24 hours. Plasma GFAP was inconsistently detected, whereas NSE was universally detectable, with higher NSE concentrations associated with delirium (risk difference 15.1% comparing 75th and 25th percentiles; 95% CI: 2.5, 27.7; p = 0.026). One year mortality appeared higher among delirious patients, 12.3% compared to 7.1%, but was not significant (p = 0.28).
Conclusions
Postoperative delirium is common in lung transplant recipients, and several potentially modifiable risk factors deserve further study to determine their associated mechanisms and predictive values.
Keywords: lung transplantation, delirium, neuron specific enolase, glial fibrillary acidic protein
Introduction
Delirium, an acute disturbance in attention and cognition, is common in critically ill patients (1–5), and was reported in 37% of lung transplant recipients in a prior study (6). Delirious patients experience significant morbidities, including prolonged mechanical ventilation, longer intensive care unit (ICU) stays, and increased risk of long-term physical and cognitive dysfunction (3, 5–10). Given the impact of delirium on lung transplant recipient outcomes, a better understanding of its incidence and potentially modifiable risk factors is needed.
Numerous studies have investigated risk factors for delirium in critically ill medical and surgical patients (1, 2, 4, 11–17); however, lung transplant recipients represent a distinct population that likely has unique delirium risk factors. One recent study found that lower mean intraoperative cerebral perfusion pressure (CPP) and primary graft dysfunction (PGD) were associated with postoperative delirium in lung transplant recipients (18), but risk factors for delirium in this population have not been well studied.
In addition, prior studies have leveraged systemic markers of cerebral injury to implicate cerebral damage in the pathophysiology of delirium in critically ill septic and cardiac surgery patients (19–22). Two of the more extensively studied markers are neuron specific enolase (NSE), a cytosolic enzyme nearly exclusive to neurons, and glial fibrillary acidic protein (GFAP), the primary intermediate filament of glial cells that is upregulated in response to neuronal injury (23–25). However, no studies have investigated whether cerebral injury is involved in the pathophysiology of delirium after lung transplantation.
The goals of this study were to determine the incidence of postoperative delirium, identify risk factors for postoperative delirium, determine if plasma concentrations of NSE and GFAP associated with postoperative delirium, and determine the long-term impact of postoperative delirium in lung transplant recipients.
Methods
Study design
We performed a retrospective cohort study of 157 patients enrolled in the Lung Transplant Outcomes Group (LTOG) study who underwent lung transplantation at the University of Pennsylvania between June 2013 and July 2016. Patients with significant pre-existing cognitive impairment were excluded. Two patients with postoperative strokes were excluded, leaving a study population of 155 patients. This study was approved by the Institutional Review Board of the University of Pennsylvania.
Outcome and risk factor definitions
We used a validated chart review method to identify delirium (26, 27). We defined delirium if a physicians’ note contained the terms “delirium”, “delirious” or “CAM positive”, or if the patient received antipsychotics. We evaluated age, gender, race, native lung disease, history of anxiety or depression based on pre-transplant psychosocial assessment (Stanford Integrated Psychosocial Assessment for Transplant, Modified Mini Screen) (28, 29), pre-transplant medications (corticosteroids, immunosuppressants, opiates, antidepressants, benzodiazepines), lung allocation score at transplantation, body mass index, cardiopulmonary bypass, operation duration, total allograft ischemic time, intraoperative hemodynamics, PGD and postoperative benzodiazepine use as potential risk factors for delirium. PGD was defined as grade 3 PGD at any time within 72 hours using the International Society for Heart and Lung Transplantation criteria (30). We defined post-operative benzodiazepine as any benzodiazepine received prior to the diagnosis of delirium. Hemodynamic measurements were extracted from the electronic medical record (EMR); mean arterial pressure (MAP) and central venous pressure (CVP) were recorded in 1-minute intervals via an arterial and pulmonary arterial catheter, respectively. Values of MAP>120 mmHg, MAP<50 mmHg and CVP>40 mmHg were reviewed using a priori selected criteria by two independent physicians (BJA and CFC) with adjudication by a third physician (JMD). CVP values ≤0 mmHg were excluded. Of 74,832 observations, MAP was consistently available with only 1,181 (1.6%) missing, but CVP was not available for 16,444 (22%) observations. Cerebral perfusion pressure (CPP) was calculated as MAP–CVP as previously described (18).
Plasma biomarker measurement
Plasma was collected 24 hours after reperfusion and stored at −80° C until thawed for analysis. NSE and GFAP concentrations were measured in duplicate using enzyme linked immunosorbent assays (R&D Systems, Minneapolis MN). Samples with visible hemolysis were excluded from NSE measurement (31). Laboratory personnel were blinded to delirium status.
Statistical analysis
Baseline comparisons were made using χ2 for categorical data and the rank-sum test for continuous data. We performed univariate logistic regression to test individual candidate risk factor associations with delirium. We assessed for nonlinear exposure-outcome relationships with inspection of locally weighted scatterplot smoothing curves, and tested whether transformations improved model fit using the likelihood ratio (LR) test (32). We calculated relative risks (RR) using regression risk analysis (33, 34). We defined confounders as any covariate that altered the beta coefficient of the risk factor-outcome association ≥ 10% (35). Risk factors with a p ≤0.20 univariate association and all confounders were included in an initial multivariable model. We a priori chose the duration of time with an intraoperative MAP<60 mmHg as our primary hemodynamic risk factor because it is the lower limit for effective cerebral autoregulation, is measured throughout the entire operation unlike CPP, and accounts for the duration of exposure (i.e. dose) and may more accurately reflect total cerebral hypoperfusion exposure. We performed a sensitivity analysis using the duration of time with an intraoperative MAP<50 mmHg, and in a secondary model used the duration of time with an intraoperative CPP<50 mmHg as our hemodynamic risk factor. We used 2×2 tables, Spearman’s rank correlation, and variance inflation factors to assess collinearity. We performed backward selection to develop a parsimonious model using LR tests. Model fit was assessed using the Hosmer-Lemeshow statistic. Next, we tested the association of plasma NSE and GFAP concentrations with delirium in separate models, assessing for confounding as described above. Lastly, to assess the impact on longer-term outcomes, we tested the association of postoperative delirium with mortality using Kaplan-Meier curves and Cox regression. Analyses were performed using Stata version 12.1 (College Station, TX). A two-sided p <0.05 was considered statistically significant.
Results
Baseline characteristics of the 155 patients are summarized in Table 1. Delirium occurred in 57 (36.8%) patients for a median of 4 days (interquartile range 2–7 days), and 67 (43.2%) patients developed grade 3 PGD at any time point. Delirious patients had higher rates of bilateral transplantation, cardiopulmonary bypass utilization and PGD, as well as longer total ischemic time, and longer durations of time with MAP<60 mmHg and CPP<50 mmHg (Table 1).
Table 1.
Characteristics of study population categorized by postoperative delirium (N=155)
| No delirium (n=98) | Delirium (n=57) | p | |
|---|---|---|---|
| Clinical variables | |||
| Age | 62.5 (57–66) | 60 (53–64) | 0.087 |
| Male gender | 61 (62%) | 37 (65)% | 0.74 |
| Caucasian Race | 88 (90%) | 49 (86%) | 0.47 |
| Native lung disease | |||
| COPD | 25 (26%) | 20 (35%) | 0.195 |
| CF/Non-CF Bronchiectasis | 11 (11%) | 6 (11%) | |
| Interstitial lung disease | 55 (56%) | 23 (40%) | |
| Other | 7 (7%) | 8 (14%) | |
| History of anxiety | 35 (36%) | 26 (46%) | 0.22 |
| History of depression | 28 (29%) | 21 (37%) | 0.29 |
| Pre-transplant medications | |||
| Corticosteroids | 44 (45%) | 25 (44%) | 0.90 |
| Immunosuppression | 12 (12%) | 8 (14%) | 0.75 |
| Opiates | 14 (14%) | 7 (12%) | 0.73 |
| Antidepressants | 29 (30%) | 17 (30%) | 0.98 |
| Benzodiazepines | 25 (25%) | 21 (37%) | 0.136 |
| Lung allocation score | 39.0 (34.7–46.8) | 41.5 (33.8–52.6) | 0.48 |
| Body mass index (kg/m2) | 26.4 (22.5–29.7) | 26.2 (21.9–29.5) | 0.84 |
| Bilateral transplant | 50 (51%) | 40 (70%) | 0.020 |
| Cardiopulmonary bypass | 20 (20%) | 23 (40%) | 0.007 |
| Duration of operation (minutes) | 463 (398–536) | 524 (434–606) | 0.004 |
| Total ischemic time (minutes) | 288 (228–355) | 328 (261–370) | 0.023 |
| Postoperative benzodiazepine treatment | 38 (39%) | 35 (61%) | 0.007 |
| Grade 3 PGD (Days 0–3) | 31 (32%) | 35 (63%) | <0.001 |
| Hemodynamic variables (n=152) | |||
| Median MAP (mmHg) | 77.5 (72–84) | 73.3 (68–82) | 0.057 |
| Lowest MAP (mmHg) | 43.5 (37–50) | 38.5 (34–44.5) | 0.015 |
| Minutes with MAP <60mmHg | 22.5 (8–82.5) | 76 (17–129) | 0.002 |
| Median CPP (mmHg) | 62 (55.5–69) | 57 (51–65) | 0.019 |
| Lowest CPP (mmHg) | 26.5 (16–35) | 20 (14–30) | 0.118 |
| Minutes with CPP <50mmHg | 39 (14–131.5) | 109 (22.5–176.5) | 0.016 |
| Plasma biomarkers (n=91) | |||
| NSE (ng/mL) | 10.3 (7.6–12.8) | 12.5 (9.7–16.6) | 0.013 |
| GFAP (ng/mL) | 0.128 (0.063–0.196) | 0.104 (0.03–0.197) | 0.43 |
| Long-term outcome | |||
| 1-year mortality | 7 (7.1%) | 7 (12.3%) | 0.28 |
Data expressed as median (interquartile range) or frequency (percent).
Definition of abbreviations: COPD = chronic obstructive pulmonary disease; CF = cystic fibrosis; PGD = primary graft dysfunction; MAP = mean arterial pressure; CPP = cerebral perfusion pressure; NSE = neuron specific enolase; GFAP = glial fibrillary acidic protein
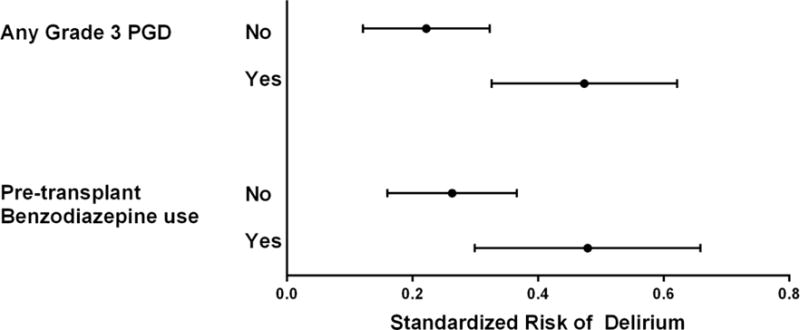
Unadjusted risk factor analyses are presented in Table 2. In our final multivariable model, age, pre-transplant benzodiazepine prescription, longer total ischemic time, longer duration of time with MAP<60 mmHg and PGD were associated with delirium (Table 3, Figure 1 Panel A–C). The association of age with delirium was nonlinear and modeling age with splines improved model fit (p < 0.001). In patients ≤50 years old, increasing age was associated with higher risk of postoperative delirium, whereas in patients >50, there was a small decreased risk of delirium with increasing age. Patients prescribed benzodiazepines prior to transplantation had a 21.6% (95% CI: 1.4, 41.7; p = 0.036) absolute increased risk of postoperative delirium. Each 30-minute increase in total ischemic time was associated with a 3.2% (95% CI: 0.3, 6.0; p=0.026) absolute increased risk of postoperative delirium, such that a total ischemic time of 365 minutes (75th percentile) was associated with an 13.3% (95% CI: 1.7, 24.9; p=0.025) absolute increased risk of postoperative delirium compared to an ischemic time of 239 minutes (25th percentile). Each 15-minute increase in the duration of time with an intraoperative MAP<60 mmHg was associated with a 2.1% (95% CI: 0.3, 3.9, p=0.021) absolute increased risk of postoperative delirium, such that a duration of 98.5 minutes (75th percentile) was associated with a 13.3% (95% CI: 1.6, 25.1; p= 0.027) absolute increased risk of postoperative delirium compared to a total duration of 8.5 minutes (25th percentile). In a sensitivity analysis using a more strict threshold of MAP<50 mmHg, the risk difference was larger but not statistically significant, with a duration of 21 minutes (75th percentile) associated with a 5.8% (95% CI: −1.4, 12.9; p = 0.11) absolute increased risk of postoperative delirium compared to a duration of 1 minute (25th percentile). Delirium and PGD were strongly associated, with patients developing PGD having a 25.2% (95% CI: 8.3, 42.0; p = 0.003) absolute increased risk of postoperative delirium. Overall, 36 patients (23%) developed both delirium and PGD, 33 (21%) developed PGD only, and 21 (14%) developed delirium only.
Table 2.
Unadjusted analysis of risk factors for postoperative delirium.
| Relative risk | 95% CI | p | |
|---|---|---|---|
| Age (per 1 year increase) | |||
| Age ≤ 50 | 1.12 | 1.01, 1.25 | 0.028 |
| Age > 50 | 0.91 | 0.85, 0.96 | 0.001 |
| Male gender | 0.93 | 0.60, 1.44 | 0.74 |
| Caucasian race | 0.80 | 0.46, 1.41 | 0.45 |
| History of anxiety | 1.29 | 0.86, 1.95 | 0.22 |
| History of depression | 1.26 | 0.83, 1.92 | 0.28 |
| Native lung disease | |||
| COPD | Reference | ||
| CF/Non-CF bronchiectasis | 0.79 | 0.39, 1.63 | 0.53 |
| Interstitial lung disease | 0.66 | 0.41, 1.07 | 0.09 |
| Other | 1.20 | 0.68, 2.13 | 0.53 |
| Pre-transplant medications | |||
| Corticosteroids | 0.97 | 0.64, 1.48 | 0.90 |
| Immunosuppressants | 1.10 | 0.62, 1.97 | 0.74 |
| Opiates | 0.89 | 0.47, 1.70 | 0.73 |
| Antidepressants | 1.01 | 0.64, 1.58 | 0.98 |
| Benzodiazepines | 1.38 | 0.91, 2.09 | 0.125 |
| Lung allocation score (per 1 point increase) | 1.00 | 0.99, 1.02 | 0.46 |
| Body mass index (per 1 kg/m2 increase) | 1.01 | 0.96, 1.05 | 0.82 |
| Cardiopulmonary bypass | 1.76 | 1.19, 2.62 | 0.005 |
| Bilateral Transplant | 1.70 | 1.06, 2.72 | 0.027 |
| Duration of the operation (per 30 minute increase) | 1.08 | 1.03, 1.14 | 0.005 |
| Total ischemic time (per 30 minute increase) | 1.10 | 1.03, 1.18 | 0.008 |
| Minutes with MAP <60mmHg (per 15 minute increase) | 1.09 | 1.03, 1.15 | 0.004 |
| Minutes with CPP <50mmHg (per 15 minute increase) | 1.06 | 1.01, 1.10 | 0.009 |
| Postoperative benzodiazepine treatment | 1.79 | 1.16, 2.74 | 0.008 |
| Grade 3 PGD | 2.25 | 1.46, 3.48 | <0.001 |
Abbreviations: COPD = chronic obstructive pulmonary disease; CF = cystic fibrosis; MAP = mean arterial pressure; CPP = cerebral perfusion pressure; PGD = primary graft dysfunction
Table 3.
Multivariable adjusted risk factors for postoperative delirium
| Relative risk | 95% CI | p value | |
|---|---|---|---|
| Age (per 1 year increase) | |||
| Age ≤ 50 | 1.20 | 1.01, 1.43 | 0.038 |
| Age > 50 | 0.93 | 0.86, 1.00 | 0.047 |
| Pre-transplant benzodiazepine prescription | 1.82 | 1.08, 3.07 | 0.025 |
| Total ischemic time (per 30 minute increase) | 1.10 | 1.01, 1.21 | 0.027 |
| Time with MAP <60mmHg (per 15 minute increase) | 1.07 | 1.00, 1.14 | 0.041 |
| Grade 3 PGD | 2.13 | 1.27, 3.58 | 0.004 |
Abbreviations: MAP = mean arterial pressure, PGD = primary graft dysfunction
Figure 1.
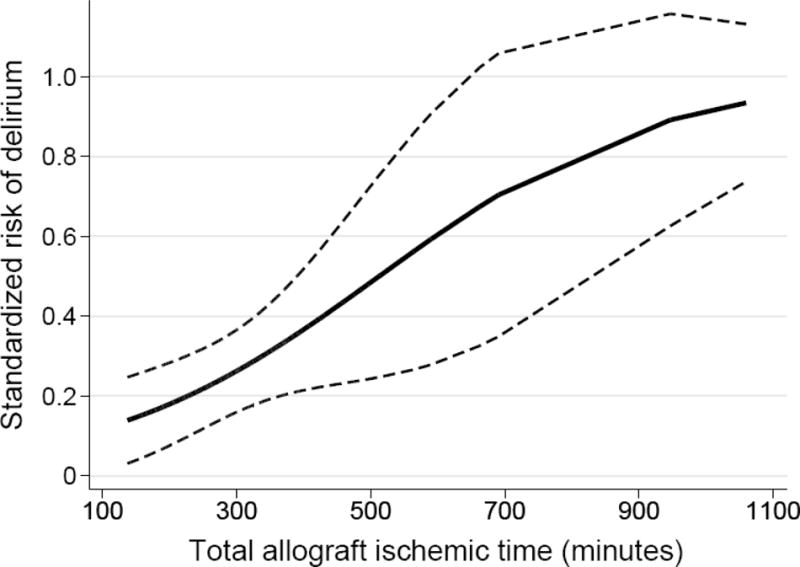
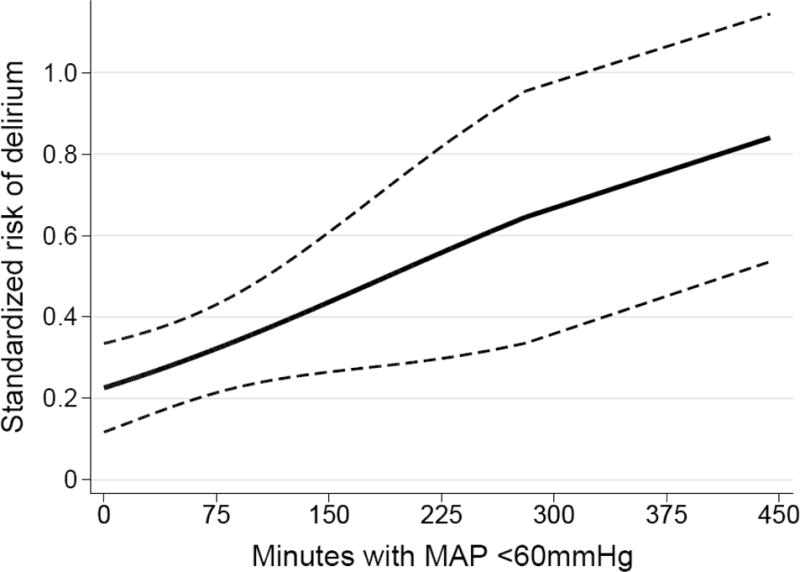
Adjusted standardized risk of postoperative delirium according to (A) total allograft ischemic time; (B) duration of time with intraoperative mean arterial pressure <60mmHg; and (C) pre-transplant benzodiazepine prescription and development of grade 3 primary graft dysfunction. Solid lines or points represent the adjusted delirium risk and dashed lines or error bars represent 95% CIs.
In our secondary model using the total time with an intraoperative CPP<50 mmHg as the primary hemodynamic risk variable, duration of time with a CPP<50 mmHg was not significantly associated with delirium (relative risk 1.04 per 15 minute increase; 95% CI: 0.99, 1.09; p=0.16). The associations of age, pre-transplant benzodiazepine prescription, total ischemic time and PGD with postoperative delirium were similar in this secondary model (Table S1).
Ninety-one (58.7%) patients had available plasma 24 hours after allograft reperfusion; no significant differences were noted between patients with and without plasma (Table S2). Only 38 (41.8%) patients had detectable plasma GFAP concentrations 24 hours after reperfusion, with no association of plasma GFAP level with postoperative delirium. All patients had detectable NSE plasma levels 24 hours after reperfusion, with a median concentration of 11.3 ug/L (interquartile range [IQR]: 8.1–14.7). Higher plasma NSE concentrations were significantly associated with postoperative delirium (Figure 2). Patients with a plasma NSE concentration at the 75th percentile (14.7 ug/L) had a 15.1% (95%CI: 2.5, 27.7; p = 0.019) absolute increased risk of postoperative delirium compared to patients with a plasma NSE concentration at the 25th percentile (8.1 ug/L). Given our sample size we were unable to adjust for our identified delirium risk factors due to overfitting; however, adjustment for the individual risk factors did not significantly alter the association and none met our criteria for confounding (Table S3).
Figure 2.
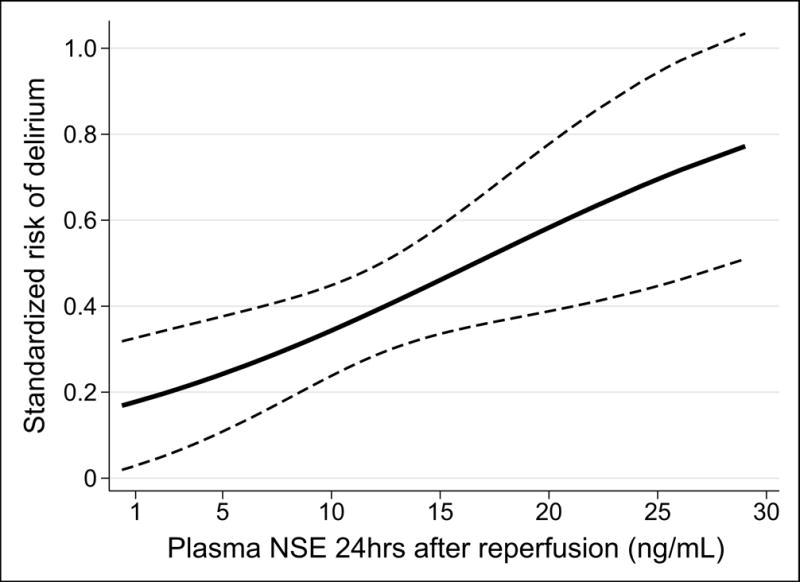
Standardized risk of postoperative delirium according to the plasma NSE concentration 24 hours after allograft reperfusion. The solid line represents the standardized risk and the dashed lines represent the 95% CIs.
In terms of long-term outcomes, patients were followed for a median of 793 (IQR: 587–1053) days after transplantation, with all patients completing at least 1-year of follow-up. Fourteen patients died within one year and we were unable to detect a significant difference in 1-year mortality between patients with and without postoperative delirium (12.3% versus 7.1%, p = 0.28). When including all follow-up data in a Cox regression analysis, we likewise could not detect a statistical significance of a higher risk point estimate (unadjusted hazard ratio 1.46; 95% CI: 0.74, 2.91; p = 0.28; Figure S1).
Discussion
Our study demonstrates that postoperative delirium is common in lung transplant recipients, occurring in approximately 37% of patients. We identified age, pre-transplant benzodiazepines, ischemic time, duration with an intraoperative MAP<60 mmHg, and PGD as independent risk factors for delirium in lung transplant recipients. Furthermore, higher plasma levels of NSE were associated with delirium, suggesting cerebral injury may be in the causal pathway of delirium in these patients.
Age is a consistent risk factor for delirium across diverse populations (2, 11–14). In our study, the association of age with delirium was non-linear. Increasing age was associated with higher delirium risk in patients aged ≤50, and lower delirium risk in patients aged >50. The selection process for older candidates may select patients who are neurologically robust and less likely to develop delirium, but our findings require validation in future studies.
We identified outpatient benzodiazepines as a novel and potentially modifiable risk factor for delirium. Benzodiazepines used during critical illness and preoperatively have been linked to delirium (1, 3, 4, 12, 16, 17, 36, 37), but the literature regarding outpatient benzodiazepines and delirium is inconclusive. Four studies report conflicting results, with the largest study reporting a doubling of delirium risk in unadjusted analyses that was not significant after adjustment (38–41). The discordant findings may be due to differences in patient populations, the type of surgery, or other factors. Future studies should seek to confirm our findings and investigate whether limiting pre-transplant benzodiazepine use reduces delirium.
We previously linked longer ischemic time with worse post-transplant cognitive function (42) and now demonstrate an association with delirium. Lung ischemia-reperfusion injury causes release of numerous cytokines (43), which may incite neuroinflammation, leading to delirium. This is consistent with studies reporting higher systemic cytokine levels in critically ill patients with delirium (44–46). Inflammatory lung injury may also incite neuroinflammation via the autonomic nervous system (47–49). Longer ischemic time has also been linked to impaired postoperative gas exchange (43), potentially contributing to delirium through hypoxemia (2). Future studies are needed to validate our findings and investigate the mechanistic underpinnings of this association.
PGD was strongly associated with delirium, consistent with the study by Smith et al. (18). Elucidating whether PGD is truly a risk factor for delirium is challenging, because PGD is defined within the first 72 hours when patients are frequently sedated and cannot be assessed for delirium, particularly patients with severe PGD. However, there are several mechanisms by which PGD may increase delirium risk. Inflammatory lung injury and hypoxia, hallmarks of PGD, may contribute to delirium as detailed above. PGD is associated with prolonged mechanical ventilation and may increase delirium risk through exposure to sedative medications or ventilator-induced brain injury (50, 51). Delirium and PGD may result from shared mechanisms, supported by our finding that ischemic time, an established PGD risk factor (52), is also a risk factor for delirium. Prospective studies are needed to further investigate this relationship, and because of potential shared pathophysiology, clinical trials for PGD should consider delirium as a secondary outcome.
The duration of time with an intraoperative MAP<60 mmHg was independently associated with delirium. Although Smith et al. reported an association of lower CPP with delirium (18), the duration of time with CPP<50 mmHg was not independently associated with delirium in our study. This is likely due to differences in the analyses between the two studies, as we included additional covariates, including ischemic time which correlated with the duration of time with CPP<50 mmHg. It may also be due to different exposure variables or differences in measurement, as CPP was frequently missing in our study. In a sensitivity analysis, the duration of time with MAP<50 mmHg was not significantly associated with delirium. This may be due to individual variability in cerebral autoregulation as seen in prior studies (53), or inadequate power given the low exposure to this degree of hypotension. Overall, our findings are consistent with the Smith et al. study and implicate cerebral hypoperfusion as a risk factor for delirium in lung transplant recipients. Since maintenance of cerebral perfusion is part of standard intraoperative care, future studies should focus on advancing methods for personalizing cerebral perfusion (53), and identifying downstream pathways that could be pharmacologically targeted to limit the impact of cerebral hypoperfusion on post-transplant outcomes.
Similar to prior studies of critically ill septic and cardiac surgery patients (19–22), we found an association of higher plasma concentrations of NSE with delirium. Our findings require validation, but suggest that cerebral injury may contribute to delirium and that measurement of NSE may be useful for quantifying cerebral injury in mechanistic studies. NSE may also be useful to identify patients at high risk for delirium who could be targeted for enrollment in clinical trials. Given the heterogeneity of delirium, NSE may be useful for differentiating subgroups of delirium with and without cerebral injury, which may respond differently to different interventions. Future studies should seek to validate our findings and investigate whether NSE has utility for prediction, delirium subphenotype definition, or as a response-indicator marker.
Delirium has been linked to long-term mortality in other critically ill patient populations (54), and the point estimate for 1-year mortality was higher in delirious patients in our study; however, we were unable to demonstrate statistical significance likely due to inadequate power. As the current study was not designed to study mortality, larger studies are needed to detect potentially relevant differences in mortality among patients who experience delirium after lung transplantation.
Our study has several limitations. Although we designed our study within the prospective LTOG cohort at our center, delirium was retrospectively determined by chart review potentially leading to under diagnosis. However, daily assessment for delirium is standard practice for our lung transplant group. In addition, our delirium incidence is strikingly similar to the prior study by Smith and colleagues that employed prospective delirium assessment, suggesting under diagnosis was minimal (18). Future studies should employ prospective delirium assessments to confirm our findings. We defined pre-transplant benzodiazepine exposure by chart review, potentially leading to exposure misclassification; future studies should obtain more detailed information about actual use and dose. Our hemodynamic variables were extracted from the EMR and are prone to measurement error; however, two physicians reviewed the data with adjudication by a third physician to limit potential measurement error. Only a subset of patients had plasma available for analysis; however, there were no significant differences between patients with and without available plasma. Although our study is the largest evaluation of delirium risk factors in lung transplant recipients to date, our study was performed within a single-center and may have limited generalizability. Given the small sample size, our model was prone to overfitting; however the final model had 9.5 events per variable suggesting the risk of overfitting was minimal (55). Lastly, although we considered a broad array of potential risk factors, coronary artery disease, cerebrovascular disease and other potential risk factors may exist and should be considered in future studies.
Conclusions
In summary, postoperative delirium is common in lung transplant recipients, occurring in over one third of patients. Age, pre-transplant benzodiazepines, ischemic time, duration of intraoperative MAP<60 mmHg, and PGD are independent risk factors for postoperative delirium in lung transplant recipients. Cerebral injury may be in the causal pathway of delirium and NSE may be an effective marker of delirium in this population. Our study highlights the need to investigate the links between ischemic time and cerebral hypoperfusion with delirium and subsequent cognitive function, and may have clinical implications, such as limiting pre-transplant benzodiazepine use.
Supplementary Material
Acknowledgments
Financial Support: The study was supported in part by National Institutes of Health grants K23HL116656 (E.C.), R03HL135227 (E.C.), K24HL115354 (J.D.C.), R01HL087115 (J.D.C.) and K23HL121406 (J.M.D.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure statement:
E.C. receives research funding from Xvivo, Inc. unrelated to the present study. J.D.C. receives research funding from GlaxoSmithKline unrelated to the present study. The remaining authors have no conflicts of interest to disclose. This study was supported by National Institutes of Health grants K23HL116656 (E.C.), K24HL115354 (J.D.C.), R01HL087115 (J.D.C.) and K23HL121406 (J.M.D.). Some results from this study were previously presented in abstract form at the 2015 American Thoracic Society International Conference.
Conflict of Interest and Disclosures: E.C. receives research funding from Xvivo, Inc. unrelated to the present study. J.D.C. receives research funding from GlaxoSmithKline unrelated to the present study. The remaining authors have no conflicts of interest to disclose.
References
- 1.Agarwal V, O’Neill PJ, Cotton BA, et al. Prevalence and risk factors for development of delirium in burn intensive care unit patients. J Burn Care Res. 2010;31:706–15. doi: 10.1097/BCR.0b013e3181eebee9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kazmierski J, Kowman M, Banach M, et al. Incidence and predictors of delirium after cardiac surgery: Results from The IPDACS Study. J Psychosom Res. 2010;69:179–85. doi: 10.1016/j.jpsychores.2010.02.009. [DOI] [PubMed] [Google Scholar]
- 3.Ouimet S, Kavanagh BP, Gottfried SB, Skrobik Y. Incidence, risk factors and consequences of ICU delirium. Intensive Care Med. 2007;33:66–73. doi: 10.1007/s00134-006-0399-8. [DOI] [PubMed] [Google Scholar]
- 4.Pandharipande P, Cotton BA, Shintani A, et al. Prevalence and risk factors for development of delirium in surgical and trauma intensive care unit patients. J Trauma. 2008;65:34–41. doi: 10.1097/TA.0b013e31814b2c4d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pandharipande PP, Girard TD, Jackson JC, et al. Long-term cognitive impairment after critical illness. N Engl J Med. 2013;369:1306–16. doi: 10.1056/NEJMoa1301372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smith PJ, Rivelli SK, Waters AM, et al. Delirium affects length of hospital stay after lung transplantation. J Crit Care. 2015;30:126–9. doi: 10.1016/j.jcrc.2014.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smith PJ, Rivelli S, Waters A, et al. Neurocognitive changes after lung transplantation. Ann Am Thorac Soc. 2014;11:1520–7. doi: 10.1513/AnnalsATS.201406-232OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lat I, McMillian W, Taylor S, et al. The impact of delirium on clinical outcomes in mechanically ventilated surgical and trauma patients. Crit Care Med. 2009;37:1898–905. doi: 10.1097/CCM.0b013e31819ffe38. [DOI] [PubMed] [Google Scholar]
- 9.Saczynski JS, Marcantonio ER, Quach L, et al. Cognitive trajectories after postoperative delirium. N Engl J Med. 2012;367:30–9. doi: 10.1056/NEJMoa1112923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brummel NE, Jackson JC, Pandharipande PP, et al. Delirium in the ICU and subsequent long-term disability among survivors of mechanical ventilation. Crit Care Med. 2014;42:369–77. doi: 10.1097/CCM.0b013e3182a645bd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gosselt AN, Slooter AJ, Boere PR, Zaal IJ. Risk factors for delirium after on-pump cardiac surgery: a systematic review. Crit Care. 2015;19:346. doi: 10.1186/s13054-015-1060-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McPherson JA, Wagner CE, Boehm LM, et al. Delirium in the cardiovascular ICU: exploring modifiable risk factors. Crit Care Med. 2013;41:405–13. doi: 10.1097/CCM.0b013e31826ab49b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zaal IJ, Devlin JW, Peelen LM, Slooter AJ. A systematic review of risk factors for delirium in the ICU. Crit Care Med. 2015;43:40–7. doi: 10.1097/CCM.0000000000000625. [DOI] [PubMed] [Google Scholar]
- 14.Guenther U, Theuerkauf N, Frommann I, et al. Predisposing and precipitating factors of delirium after cardiac surgery: a prospective observational cohort study. Annals of surgery. 2013;257:1160–7. doi: 10.1097/SLA.0b013e318281b01c. [DOI] [PubMed] [Google Scholar]
- 15.Koster S, Hensens AG, Schuurmans MJ, van der Palen J. Risk factors of delirium after cardiac surgery: a systematic review. Eur J Cardiovasc Nurs. 2011;10:197–204. doi: 10.1016/j.ejcnurse.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 16.Dubois MJ, Bergeron N, Dumont M, Dial S, Skrobik Y. Delirium in an intensive care unit: a study of risk factors. Intensive Care Med. 2001;27:1297–304. doi: 10.1007/s001340101017. [DOI] [PubMed] [Google Scholar]
- 17.Van Rompaey B, Elseviers MM, Schuurmans MJ, Shortridge-Baggett LM, Truijen S, Bossaert L. Risk factors for delirium in intensive care patients: a prospective cohort study. Crit Care. 2009;13:R77. doi: 10.1186/cc7892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smith PJ, Blumenthal JA, Hoffman BM, et al. Reduced Cerebral Perfusion Pressure during Lung Transplant Surgery Is Associated with Risk, Duration, and Severity of Postoperative Delirium. Ann Am Thorac Soc. 2016;13:180–7. doi: 10.1513/AnnalsATS.201507-454OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Herrmann M, Ebert AD, Galazky I, Wunderlich MT, Kunz WS, Huth C. Neurobehavioral outcome prediction after cardiac surgery: role of neurobiochemical markers of damage to neuronal and glial brain tissue. Stroke. 2000;31:645–50. doi: 10.1161/01.str.31.3.645. [DOI] [PubMed] [Google Scholar]
- 20.Anderson BJ, Reilly JP, Shashaty MGS, et al. Admission plasma levels of the neuronal injury marker neuron-specific enolase are associated with mortality and delirium in sepsis. J Crit Care. 2016;36:18–23. doi: 10.1016/j.jcrc.2016.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ramlawi B, Rudolph JL, Mieno S, et al. Serologic markers of brain injury and cognitive function after cardiopulmonary bypass. Annals of surgery. 2006;244:593–601. doi: 10.1097/01.sla.0000239087.00826.b4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baranyi A, Rothenhausler HB. The impact of S100b and persistent high levels of neuron-specific enolase on cognitive performance in elderly patients after cardiopulmonary bypass. Brain Inj. 2013;27:417–24. doi: 10.3109/02699052.2012.750751. [DOI] [PubMed] [Google Scholar]
- 23.Taylor CB, Royds JA, Parsons MA, Timperley WR. Diagnostic aspects of enolase isozymes. Isozymes. 1983;11:95–119. [PubMed] [Google Scholar]
- 24.Royds JA, Parsons MA, Taylor CB, Timperley WR. Enolase isoenzyme distribution in the human brain and its tumours. The Journal of pathology. 1982;137:37–49. doi: 10.1002/path.1711370105. [DOI] [PubMed] [Google Scholar]
- 25.Hol EM, Pekny M. Glial fibrillary acidic protein (GFAP) and the astrocyte intermediate filament system in diseases of the central nervous system. Curr Opin Cell Biol. 2015;32:121–30. doi: 10.1016/j.ceb.2015.02.004. [DOI] [PubMed] [Google Scholar]
- 26.Inouye SK, Leo-Summers L, Zhang Y, Bogardus ST, Jr, Leslie DL, Agostini JV. A chart-based method for identification of delirium: validation compared with interviewer ratings using the confusion assessment method. J Am Geriatr Soc. 2005;53:312–8. doi: 10.1111/j.1532-5415.2005.53120.x. [DOI] [PubMed] [Google Scholar]
- 27.Puelle MR, Kosar CM, Xu G, et al. The Language of Delirium: Keywords for Identifying Delirium from Medical Records. J Gerontol Nurs. 2015;41:34–42. doi: 10.3928/00989134-20150723-01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maldonado JR, Dubois HC, David EE, et al. The Stanford Integrated Psychosocial Assessment for Transplantation (SIPAT): a new tool for the psychosocial evaluation of pre-transplant candidates. Psychosomatics. 2012;53:123–32. doi: 10.1016/j.psym.2011.12.012. [DOI] [PubMed] [Google Scholar]
- 29.Alexander MJ, Haugland G, Lin SP, Bertollo DN, McCorry FA. Mental Health Screening in Addiction, Corrections and Social Service Settings: Validating the MMS. Int J Ment Health Addiction. 2008;6:105–19. [Google Scholar]
- 30.Snell GI, Yusen RD, Weill D, et al. Report of the ISHLT Working Group on Primary Lung Graft Dysfunction, part I: Definition and grading-A 2016 Consensus Group statement of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant. 2017;36:1097–103. doi: 10.1016/j.healun.2017.07.021. [DOI] [PubMed] [Google Scholar]
- 31.Kato K, Asai R, Shimizu A, Suzuki F, Ariyoshi Y. Immunoassay of three enolase isozymes in human serum and in blood cells. Clin Chim Acta. 1983;127:353–63. doi: 10.1016/0009-8981(83)90162-6. [DOI] [PubMed] [Google Scholar]
- 32.Cleveland WS. Robust Locally Weighted Regression and Smoothing Scatterplots. J Am Stat Assoc. 1979;74:829–36. [Google Scholar]
- 33.Kleinman LC, Norton EC. What’s the Risk? A simple approach for estimating adjusted risk measures from nonlinear models including logistic regression. Health Serv Res. 2009;44:288–302. doi: 10.1111/j.1475-6773.2008.00900.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Norton EC, Miller MM, Kleinman LC. Computing adjusted risk ratios and risk differences in Stata. Stata J. 2013;13:492–509. [Google Scholar]
- 35.Maldonado G, Greenland S. Simulation study of confounder-selection strategies. Am J Epidemiol. 1993;138:923–36. doi: 10.1093/oxfordjournals.aje.a116813. [DOI] [PubMed] [Google Scholar]
- 36.Pandharipande P, Shintani A, Peterson J, et al. Lorazepam is an independent risk factor for transitioning to delirium in intensive care unit patients. Anesthesiology. 2006;104:21–6. doi: 10.1097/00000542-200601000-00005. [DOI] [PubMed] [Google Scholar]
- 37.Marcantonio ER, Juarez G, Goldman L, et al. The relationship of postoperative delirium with psychoactive medications. JAMA. 1994;272:1518–22. [PubMed] [Google Scholar]
- 38.Bohner H, Hummel TC, Habel U, et al. Predicting delirium after vascular surgery: a model based on pre- and intraoperative data. Annals of surgery. 2003;238:149–56. doi: 10.1097/01.sla.0000077920.38307.5f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kudoh A, Takase H, Takahira Y, Takazawa T. Postoperative confusion increases in elderly long-term benzodiazepine users. Anesth Analg. 2004;99:1674–8. doi: 10.1213/01.ANE.0000136845.24802.19. table of contents. [DOI] [PubMed] [Google Scholar]
- 40.Litaker D, Locala J, Franco K, Bronson DL, Tannous Z. Preoperative risk factors for postoperative delirium. Gen Hosp Psychiatry. 2001;23:84–9. doi: 10.1016/s0163-8343(01)00117-7. [DOI] [PubMed] [Google Scholar]
- 41.Weed HG, Lutman CV, Young DC, Schuller DE. Preoperative identification of patients at risk for delirium after major head and neck cancer surgery. Laryngoscope. 1995;105:1066–8. doi: 10.1288/00005537-199510000-00011. [DOI] [PubMed] [Google Scholar]
- 42.Cohen DG, Christie JD, Anderson BJ, et al. Cognitive function, mental health, and health-related quality of life after lung transplantation. Ann Am Thorac Soc. 2014;11:522–30. doi: 10.1513/AnnalsATS.201311-388OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thabut G, Mal H, Cerrina J, et al. Graft ischemic time and outcome of lung transplantation: a multicenter analysis. Am J Respir Crit Care Med. 2005;171:786–91. doi: 10.1164/rccm.200409-1248OC. [DOI] [PubMed] [Google Scholar]
- 44.Girard TD, Ware LB, Bernard GR, et al. Associations of markers of inflammation and coagulation with delirium during critical illness. Intensive Care Med. 2012;38:1965–73. doi: 10.1007/s00134-012-2678-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McGrane S, Girard TD, Thompson JL, et al. Procalcitonin and C-reactive protein levels at admission as predictors of duration of acute brain dysfunction in critically ill patients. Critical Care. 2011;15:R78. doi: 10.1186/cc10070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kazmierski J, Banys A, Latek J, Bourke J, Jaszewski R. Raised IL-2 and TNF-alpha concentrations are associated with postoperative delirium in patients undergoing coronary-artery bypass graft surgery. Int Psychogeriatr. 2014;26:845–55. doi: 10.1017/S1041610213002378. [DOI] [PubMed] [Google Scholar]
- 47.Fries M, Bickenbach J, Henzler D, et al. S-100 protein and neurohistopathologic changes in a porcine model of acute lung injury. Anesthesiology. 2005;102:761–7. doi: 10.1097/00000542-200504000-00011. [DOI] [PubMed] [Google Scholar]
- 48.Gonzalvo R, Marti-Sistac O, Blanch L, Lopez-Aguilar J. Bench-to-bedside review: brain-lung interaction in the critically ill–a pending issue revisited. Crit Care. 2007;11:216. doi: 10.1186/cc5930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Goehler LE, Gaykema RP, Hansen MK, Anderson K, Maier SF, Watkins LR. Vagal immune-to-brain communication: a visceral chemosensory pathway. Auton Neurosci. 2000;85:49–59. doi: 10.1016/S1566-0702(00)00219-8. [DOI] [PubMed] [Google Scholar]
- 50.Christie JD, Sager JS, Kimmel SE, et al. Impact of primary graft failure on outcomes following lung transplantation. Chest. 2005;127:161–5. doi: 10.1378/chest.127.1.161. [DOI] [PubMed] [Google Scholar]
- 51.Gonzalez-Lopez A, Lopez-Alonso I, Aguirre A, et al. Mechanical ventilation triggers hippocampal apoptosis by vagal and dopaminergic pathways. Am J Respir Crit Care Med. 2013;188:693–702. doi: 10.1164/rccm.201304-0691OC. [DOI] [PubMed] [Google Scholar]
- 52.Diamond JM, Lee JC, Kawut SM, et al. Clinical risk factors for primary graft dysfunction after lung transplantation. Am J Respir Crit Care Med. 2013;187:527–34. doi: 10.1164/rccm.201210-1865OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Brady K, Joshi B, Zweifel C, et al. Real-time continuous monitoring of cerebral blood flow autoregulation using near-infrared spectroscopy in patients undergoing cardiopulmonary bypass. Stroke. 2010;41:1951–6. doi: 10.1161/STROKEAHA.109.575159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pisani MA, Kong SY, Kasl SV, Murphy TE, Araujo KL, Van Ness PH. Days of delirium are associated with 1-year mortality in an older intensive care unit population. Am J Respir Crit Care Med. 2009;180:1092–7. doi: 10.1164/rccm.200904-0537OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Peduzzi P, Concato J, Kemper E, Holford TR, Feinstein AR. A simulation study of the number of events per variable in logistic regression analysis. J Clin Epidemiol. 1996;49:1373–9. doi: 10.1016/s0895-4356(96)00236-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


