Abstract
Background
Triclosan, an antimicrobial agent used in some consumer products, reduces endogenous thyroid hormone concentrations in rodents. Despite ubiquitous triclosan exposure and the importance of thyroid hormones for normal fetal development, few human studies have examined the impact of triclosan exposure on maternal, neonatal, or child thyroid hormones.
Methods
In the HOME Study, a prospective cohort from Cincinnati, OH, we measured urinary triclosan concentrations up to three times in pregnant women between 16 weeks and delivery, and up to three times in children between age 1–3 years. We quantified serum concentrations of thyroid stimulating hormone and total and free thyroxine and triiodothyronine in mothers at 16-weeks gestation (n=202), neonates at delivery (n=274), and children at age 3 years (n=153). We estimated covariate-adjusted differences in thyroid hormones with a 10-fold increase in triclosan using linear regression and multiple informants models.
Results
Triclosan was not associated with thyroid hormones during pregnancy. We observed a few associations of triclosan concentrations with thyroid hormone concentrations in neonates at delivery and children at age 3 years. Higher gestational triclosan, particularly around the time of delivery, was associated with lower cord serum total thyroxine (β:0.3 μg/dL; 95% CI:−0.6, −0.0). Childhood triclosan, particularly at age 1 year, was positively associated with total thyroxine at age 3 years (β:0.7 μg/dL; 95% CI:0.3, 1.2).
Conclusion
Our findings suggest that triclosan exposure may influence some features of neonatal and early child thyroid function. Given the large number of comparisons we made, these findings should be replicated in other cohorts.
Introduction
Triclosan is an antimicrobial agent used in some toothpastes, personal care products, soaps, cleaning supplies, and medical devices (Rodricks et al., 2010). Exposure may occur either through oral ingestion (e.g., toothpaste) or dermal absorption (e.g., soaps or personal care products) (Allmyr et al., 2006; Dann and Hontela, 2011). Triclosan has a biological half-life <24 hours and is predominately excreted in the urine as a glucuronide or sulfate conjugate (Sandborgh-Englund et al., 2006). Triclosan exposure is ubiquitous in many countries with average urinary concentrations ranging from 6–30 ng/mL and <1–32 ng/mL among pregnant women and children, respectively (Casas et al., 2011; Li et al., 2013; Stacy et al., 2017; Wolff et al., 2010; Woodruff et al., 2011).
Triclosan causes reductions in serum thyroxine (T4) concentrations in rodents by increasing hepatic catabolism of endogenous thyroid hormones (Johnson et al., 2016; Paul et al., 2012; Paul et al., 2010). Decreases in T4 during fetal neurodevelopment may cause reduced cognitive abilities or increased risk of behavior problems in children (Ghassabian et al., 2011; Henrichs et al., 2013). Only a few epidemiological studies examining triclosan exposure and thyroid function in adolescents or adults have been conducted, with inconsistent findings (Cullinan et al., 2012; Geens et al., 2015; Koeppe et al., 2013). In a study of pregnant women, 3rd trimester urinary triclosan concentrations were inversely associated with maternal free T4 concentrations in the 3rd trimester and cord serum free triiodothyronine (T3) concentrations (Wang et al., 2017). However, no studies have examined whether urinary triclosan concentrations are associated with thyroid hormones in the first or second trimester of pregnancy, when the fetus is reliant on the mother for T4 (de Escobar et al., 2004). Moreover, we are not aware of any prospective studies examining the impact of triclosan exposure on thyroid function during early childhood, a critical period of neurodevelopment. Finally, no studies have examined if there are unique windows of heightened susceptibility to triclosan exposure.
To address these important research gaps, we examined the association of repeated gestational and childhood urinary triclosan concentrations with maternal, neonatal, and child serum thyroid hormone concentrations among women and their children in the Health Outcomes and Measures of the Environment (HOME) Study. We also sought to identify whether there were windows of heightened susceptibility to triclosan exposure.
Methods
Study Participants
We used data from the HOME Study, a prospective cohort study designed to quantify the health effects of early life exposure to prevalent environmental chemicals. Details regarding eligibility, recruitment, and follow-up have been previously published (Braun et al., 2016). Briefly, we recruited pregnant women from seven prenatal clinics associated with three hospitals in the Cincinnati, Ohio area from March 2003 to January 2006. The eligibility criteria at enrollment were: 1) 16±3 weeks gestation, 2) ≥18 years old, 3) living in a home built before 1978, 4) no history of HIV infection, and 5) not taking any medications for seizure or thyroid disorders. After research assistants explained study protocols, all women provided written informed consent for themselves and their children. The institutional review boards of Cincinnati Children’s Hospital Medical Center and the cooperating delivery hospitals approved this study.
Urinary Triclosan Concentration Measurements during Pregnancy
Women provided up to two urine samples during their prenatal care clinic visits around 16 and 26 weeks of pregnancy and another within 48 hours after delivery. Almost all women (97%) provided at least two urine samples. We collected urine samples from children during study visits completed between 2004 and 2009, which included clinic or home visits at ages 1, 2, and 3 years. Eighty-five percent of children provided at least one urine sample; among those 71% provided at least two samples.
Caregivers were asked to wipe their child’s genital area with a triclosan-free towelette before urine collection. We collected urine using Kendall abdominal pads placed inside the diaper for non-toilet trained children, a training potty lined with inserts for children who were being toilet trained, or specimen cups for children who were toilet trained. We followed the recommendations of Ye et al. (2013) to minimize the potential for external contamination during urine sample collection, storage, and analysis (Stacy et al., 2017; Ye et al., 2013). Details regarding our quality control procedures are available in the Supplemental Methods and Supplemental Table 1.
All samples were refrigerated until they were processed (≤24 hours), after which they were stored at or below −20°C until shipped on dry ice to Centers for Disease Control and Prevention (CDC) for analysis. Total urine triclosan concentrations (free + conjugated) were measured at the CDC laboratories using previously described analytic chemistry methods and the coefficient of variation (CV) for the assay ranged from 5.2 to 9.3% for low and high concentration quality control samples (Ye et al., 2008). The limit of detection (LOD) for the assay was 2.3 ng/mL and concentrations below the LOD were assigned a value of the LOD/√2. To account for urine dilution, we measured urinary creatinine concentrations using a kinetic Jaffe reaction, and triclosan concentrations were divided by creatinine concentrations and multiplied by 100 to yield units of μg triclosan/g creatinine.
Maternal, Neonatal, and Child Serum Thyroid Hormone Concentrations
We collected venous blood from mothers at approximately 16 weeks gestation, venous umbilical cord blood from neonates at delivery, and venous blood from children at approximately age 3 years. Blood samples collected during pregnancy and at age 3 years were collected at the same time as the urine samples that we quantified triclosan in. We separated serum from clotted blood and stored it at −80 °C until analysis. The clinical chemistry laboratory in the Department of Laboratory Medicine at the University of Washington quantified thyroid stimulating hormone (TSH), total and free T4 (TT4 and fT4), total and free T3 (TT3 and fT3), and thyroid antibodies (thyroid peroxidase [TPOAb] and thyroglobulin antibodies [TgAb]) concentrations in maternal and cord sera using an Access2 automated clinical immunoassay analyzer (Beckman Coulter Inc.). Because the volume of serum samples from age 3 years was limited, we prioritized thyroid hormone assays in these samples as follows: TSH > fT4 > TT4 > fT3> TT3 > thyroid antibodies. This resulted in varying sample sizes for analyses of thyroid hormones at age 3 years. The coefficient of variation for internal quality control samples ranged from 2.0 to 10%. Masked replicate quality control samples also had low coefficients of variation (≤8.5%).
Covariates
We considered adjusting for potential confounders that might be associated with both urinary triclosan concentrations and maternal, neonatal, or child serum thyroid hormone function. We used a directed acyclic graph (DAG) to select confounders that were not causal intermediates and associated with both urinary triclosan concentrations and thyroid function (Supplemental Figures 1–3). We used previously published results from this cohort to identify covariates associated with maternal and child triclosan concentrations (Stacy et al., 2017). Trained research assistants collected covariate information using standardized computer-assisted interviews and medical chart reviews. Sociodemographic covariates included maternal race, age, education, marital status, household income during pregnancy, and child sex and race. We abstracted maternal weight and height at 16 weeks gestation from medical records and measured child weight and height at age 3 years. These were used to calculate maternal and child body mass index (BMI). We measured exposure to tobacco smoke using serum cotinine concentrations, a sensitive and specific biomarker of nicotine (Bernert et al., 2009). Serum cotinine concentrations were measured up to three times during pregnancy and at delivery, as well as up to three times from ages 1–3 years. We calculated the average of available measures for gestational and childhood periods separately. Finally, we assessed urinary iodine concentrations in a subset of women with sufficient urine collected at 16 (3%) or 26 (97%) weeks gestation with an Agilent 7500cx Inductively Coupled Plasma-Mass Spectrometer at the Trace Element Analysis Facility at Dartmouth College (Caldwell et al., 2003). The LOD was 0.5 μg/L, with an average CV% across quality control replicates of 8%.
Statistical Analyses
We began our analyses by describing gestational and childhood urinary triclosan and serum thyroid hormone concentrations at each study visit. We calculated intraclass correlation coefficients (ICC) between repeated measures of thyroid hormones during the gestational and childhood periods. We calculated average gestational and childhood creatinine-standardized urinary triclosan concentrations by taking the mean of available log10-transformed creatinine-standardized urinary triclosan concentrations among mothers and children, respectively. If a mother or child only had one sample, we used that as their mean. We calculated the Pearson correlation coefficient of average gestational and childhood triclosan concentrations. Next, we described maternal, neonatal, or child TSH concentrations according to covariates.
We examined the shape of the dose-response relationship of log10-transformed 16-week, average gestational, or average childhood urinary triclosan concentrations with serum thyroid hormone concentrations using a 3-knot restricted cubic polynomial spline (Desquilbet and Mariotti, 2010). When associations were linear, we used multivariable linear regression models to estimate the change in serum thyroid hormone concentrations per 10-fold increase in urinary triclosan concentrations. We ln-transformed serum TSH concentrations and calculated the percent change in TSH concentrations with increasing urinary triclosan concentrations. We modeled serum T4 and T3 concentrations on their original scales.
We used linear regression to estimate the unadjusted and covariate-adjusted differences in thyroid hormone concentrations per 10-fold increase in urinary triclosan concentrations. For the analyses of maternal thyroid hormones, we used 16 week triclosan concentrations and adjusted these models for maternal age, race, BMI at 16 weeks gestation, household income, and gestational age at the time of sample collection. For analyses of cord serum thyroid hormone concentrations, we examined the average gestational triclosan concentrations. We adjusted these models for maternal age, race, household income, BMI at 16 weeks, and child sex. For analyses of thyroid hormones at age 3 years, we examined the average gestational and average childhood triclosan concentrations in separate models. We adjusted these models for household income, child race, BMI at age 3 years, child sex, and age at 3 years.
Finally, we used a multiple informants model to test whether there were periods of heightened susceptibility to triclosan exposure using our six repeated urinary triclosan concentrations from mothers and children (Sanchez et al., 2011). The multiple informants model treats individual triclosan concentrations as informants and simultaneously estimates associations between each individual triclosan concentration and a given thyroid hormone in the same statistical model. As described by Sanchez et al. (2011), the model takes the form:
| (Equation 1) |
Where E(Yi) is the mean thyroid hormone concentration for the i-th participant, β0k is the intercept for the association between thyroid hormone and triclosan concentrations measured at the k-th visit, β1k is the slope of the association between thyroid hormone and triclosan concentrations measured at the k-th visit, and β2k is the association between thyroid hormone concentrations and covariates. The model estimates the association between thyroid hormone and urinary triclosan concentrations at each time point (i.e., β11,β12) using generalized estimating equations with a working independence correlation matrix. In addition, we can statistically tests the null hypothesis that the triclosan coefficients at each visit are equal (i.e., β11 =β12 = … =β1k) vs. the alternative hypothesis that at least one β1k differs from the others. The model does not adjust for triclosan concentrations in the other periods.
Modifiers
We conducted two sets of analyses to examine potential effect measure modification of triclosan-thyroid associations. First, we examined if the associations between triclosan and thyroid hormones at delivery or age 3 years were modified by child sex because we and others have previously observed sex-specific effects of other environmental chemicals (Chevrier et al., 2013; Romano et al., 2015). We did this by including a product interaction term between urinary triclosan concentrations and children’s sex. If the p-value for this interaction term was <0.10, we considered modification to be present and estimated sex-specific associations. Second, in light of the importance of iodine in thyroid hormone production and because we previously observed that urinary iodine concentrations modified the association between gestational urinary bisphenol A and neonatal serum thyroid hormone concentrations, we examined whether the associations of gestational urinary triclosan concentrations with maternal or cord serum thyroid hormones concentrations were modified by creatinine-standardized maternal urinary iodine concentrations (insufficient: <150 μg/g vs. sufficient: ≥150 μg/g) (Romano et al., 2015). Finally, we conducted an exploratory analysis examining whether urinary triclosan concentrations were associated with TPO antibody concentrations.
Sensitivity Analyses
We examined whether any of our notable associations were attenuated when we adjusted for maternal urinary iodine concentrations since iodine may be an important confounder of the association between environmental chemicals and thyroid function (Chevrier, 2013). In addition, we adjusted for average urinary BPA concentrations at 16 and 26 weeks and delivery because we previously observed that gestational BPA concentrations were associated with both gestational triclosan concentrations and cord blood TSH concentrations in this cohort (Romano et al., 2015; Stacy et al., 2017). Second, we examined whether our associations were confounded by compromised thyroid function by excluding women, neonates, or children with clinically significant elevations in thyroid peroxidase antibodies (>9.0 IU/mL), a marker of compromised thyroid function. Finally, because labor and delivery complications could lead to both compromised neonatal thyroid function and medical interventions with triclosan exposure, we adjusted for mode of delivery and excluded neonates who were admitted to the neonatal intensive care unit (NICU).
Results
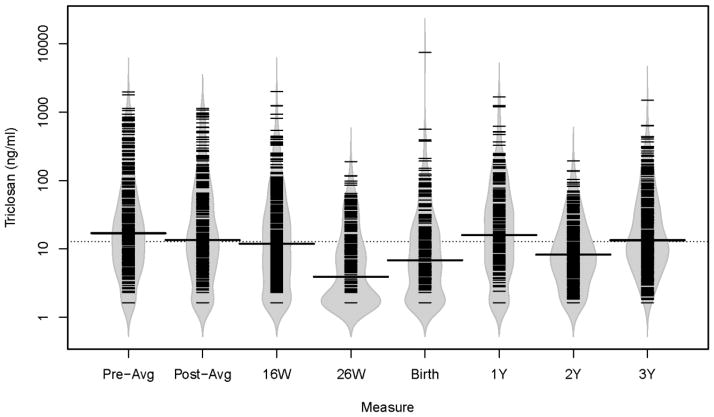
A total of 350 mother-child pairs were included in at least one of our primary analyses: 203 for 16-week thyroid hormones, 277 for cord blood thyroid hormones, and 153 for age 3 year thyroid hormones. Average gestational urinary triclosan concentrations were higher than average childhood urinary triclosan concentrations (medians: 14 vs. 8.1 ng/mL) (Figure 1, Supplemental Table 2). Average gestational and childhood urinary concentrations were weakly correlated (Pearson r=0.22, p-value=0.0001). Maternal urinary triclosan concentrations declined slightly from 16 weeks gestation to delivery (17 to 12 ng/mL) (Figure 1, Supplemental Table 2). In contrast, children’s median urinary triclosan concentrations increased from age 1 to 3 years (4.2 to 15 ng/mL). Individual gestational (ICC=0.53) and childhood (ICC=0.56) urinary triclosan concentrations had fair reproducibility (Stacy et al., 2017).
Figure 1.
Bean plots of urinary triclosan concentrations during pregnancy, at delivery, and during the first three years of life among mother-child pairs from the HOME Study.
Dotted line represents the overall median urinary triclosan concentration and solid line in each bean plot represents the median urinary triclosan concentration for that visit. The gray area is a density function of triclosan concentrations at each visit, with individual observations indicated by the narrow black lines. W=weeks of gestation, Y= age in years.
Few women, neonates, or children had clinically elevated TSH concentrations at 16 weeks gestation (>3.0 mIU/L: n=14, 6.9%), delivery (>20 mIU/L: n=12, 4.3%), or age 3 years (>5 mIU/L: n=2, 1.3%), respectively (Table 1) (Alexander et al., 2017; Manglik et al., 2005). TSH concentrations in mothers at 16 weeks and their neonates at delivery were weakly correlated (Pearson r=0.22, p-value<0.05) and children’s TSH concentrations at delivery and age 3 years were also weakly correlated (Pearson r=0.19, p-value<0.05). There were no other correlations between repeated thyroid hormone concentrations.
Table 1.
Univariate description of serum thyroid hormone concentrations in maternal serum at 16 weeks gestation, neonatal cord serum, and child serum at age 3 years among HOME Study women and their children
| Thyroid Hormone and Timing | N | Mean | SD | Min | 5th | 25th | Median | 75th | 95th | Max |
|---|---|---|---|---|---|---|---|---|---|---|
| 16 Week | ||||||||||
| TSH (mIU/mL) | 203 | 1.5 | 0.9 | 0.9 | 0.3 | 0.9 | 1.3 | 2.0 | 3.2 | 6.3 |
| TT4 (μg/dL) | 203 | 10 | 1.9 | 1.9 | 7.6 | 9.1 | 10 | 12 | 13 | 16 |
| TT3 (ng/dL) | 203 | 161 | 25 | 25 | 124 | 143 | 157 | 176 | 203 | 243 |
| fT4 (ng/dL) | 203 | 0.7 | 0.1 | 0.1 | 0.6 | 0.6 | 0.7 | 0.8 | 0.9 | 1.0 |
| fT3 (pg/mL) | 203 | 3.2 | 0.3 | 0.3 | 2.7 | 3.0 | 3.2 | 3.4 | 3.7 | 4.2 |
| Delivery | ||||||||||
| TSH (mIU/mL) | 277 | 8.5 | 6.1 | 1.0 | 2.9 | 5.1 | 7.1 | 9.9 | 18 | 49 |
| TT4 (μg/dL) | 272 | 9.6 | 1.8 | 2.4 | 6.6 | 8.5 | 9.6 | 11 | 12 | 15 |
| TT3 (ng/dL) | 277 | 51 | 19 | 7.0 | 30 | 40 | 49 | 58 | 83 | 183 |
| fT4 (ng/dL) | 277 | 1.0 | 0.2 | 0.4 | 0.8 | 0.9 | 1.0 | 1.1 | 1.2 | 1.5 |
| fT3 (pg/mL) | 275 | 1.7 | 0.3 | 0.7 | 1.3 | 1.4 | 1.6 | 1.8 | 2.2 | 3.0 |
| 3 Years | ||||||||||
| TSH (mIU/mL) | 153 | 2.2 | 1.0 | 0.5 | 1.0 | 1.5 | 1.9 | 2.7 | 4.4 | 5.6 |
| TT4 (μg/dL) | 125 | 8.8 | 1.2 | 5.6 | 7.1 | 7.9 | 8.8 | 9.5 | 11 | 13 |
| TT3 (ng/dL) | 91 | 149 | 25 | 96 | 116 | 129 | 143 | 169 | 197 | 212 |
| fT4 (ng/dL) | 136 | 0.9 | 0.1 | 0.7 | 0.7 | 0.9 | 0.9 | 1.0 | 1.1 | 1.3 |
| fT3 (pg/mL) | 75 | 4.3 | 0.5 | 3.3 | 3.5 | 4.0 | 4.3 | 4.6 | 5.3 | 5.6 |
Serum TSH concentrations at 16 weeks gestation were higher among women who were older or obese, or had higher income or lower urinary iodine concentrations (Supplemental Table 3). Cord serum TSH concentrations were higher in neonates born to women who were overweight, but not obese, unexposed to tobacco smoke, had higher income, or delivered at ≥37 weeks gestation (Supplemental Table 3). Serum TSH concentrations at age 3 years were not substantially different across categories of covariates (Supplemental Table 3).
We did not observe evidence of non-linear associations between urinary triclosan concentrations and thyroid hormones (all non-linearity p-values≥0.17). Generally, our results were similar before and after adjustment for covariates (Table 2). We observed no associations between urinary triclosan and serum thyroid hormone concentrations among pregnant women at 16 weeks gestation (Table 2).
Table 2.
Unadjusted and adjusted difference in serum thyroid hormone concentrations at 16 weeks gestation, delivery, or age 3 years with a 10-fold increase in average gestational or average childhood urinary triclosan concentrations among HOME Study women and their children
| Thyroid Hormone and Timing of Measurement | N | Gestational β (95% CI)a | Childhood β (95% CI)b | ||
|---|---|---|---|---|---|
|
| |||||
| Unadjusted | Adjusted | Unadjusted | Adjusted | ||
| 16 Weekc | |||||
| TSH (%)d | 202 | −16 (−40, 17) | −21 (−44, 11) | N/A | N/A |
| TT4 (μg/dL) | 202 | 0.2 (−0.2, 0.6) | 0.3 (−0.1, 0.6) | N/A | N/A |
| TT3 (ng/dL) | 202 | −4.2 (−9, 0.6) | −2 (−6.7, 2.7) | N/A | N/A |
| fT4 (ng/dL) | 202 | 0.01 (−0.01, 0.03) | 0.01 (−0.01, 0.03) | N/A | N/A |
| fT3 (pg/mL) | 202 | −0.06 (−0.12, 0) | −0.04 (−0.1, 0.02) | N/A | N/A |
| Deliverye | |||||
| TSH (%)d | 274 | 29 (−1, 67) | 15 (−14, 52) | N/A | N/A |
| TT4 (μg/dL) | 269 | −0.2 (−0.6, 0.1) | −0.3 (−0.7, 0.1) | N/A | N/A |
| TT3 (ng/dL) | 274 | −1.5 (−5.2, 2.2) | −2.4 (−6.3, 1.6) | N/A | N/A |
| fT4 (ng/dL) | 274 | 0 (−0.03, 0.03) | 0 (−0.03, 0.03) | N/A | N/A |
| fT3 (pg/mL) | 272 | −0.04 (−0.1, 0.02) | −0.05 (−0.11, 0.02) | N/A | N/A |
| 3 Yearsf | |||||
| TSH (%)d | 153 | 40 (5, 87) | 32 (−3, 80) | 12 (−26, 69) | 2 (−34, 56) |
| TT4 (μg/dL) | 125 | 0 (−0.3, 0.3) | 0 (−0.4, 0.3) | 0.4 (−0.1, 0.9) | 0.4 (−0.1, 0.9) |
| TT3 (ng/dL) | 91 | −6.9 (−15.8, 2) | −6.1 (−15.8, 3.6) | 0.8 (−11.8, 13.4) | −0.8 (−14.4, 12.8) |
| fT4 (ng/dL) | 136 | 0 (−0.03, 0.04) | 0 (−0.04, 0.04) | 0.06 (0.01, 0.11) | 0.06 (0.01, 0.11) |
| fT3 (pg/mL) | 75 | −0.08 (−0.27, 0.12) | −0.05 (−0.27, 0.17) | 0.02 (−0.25, 0.3) | 0.12 (−0.19, 0.44) |
For the 16 week thyroid hormones, the gestational coefficient represents the difference in 16 week thyroid hormone concentrations with a 10-fold increase in creatinine-standardized urinary triclosan concentrations at 16 weeks gestation. For the other thyroid hormone concentrations, the gestational coefficient represents the difference in thyroid hormone concentrations with a 10-fold increase in the average of log10-transformed creatinine-standardized urinary triclosan concentrations at 16 and 26 weeks and birth.
The childhood coefficient represents the difference in thyroid hormone concentrations with a 10-fold increase in the average of log10-transformed creatinine-standardized urinary triclosan concentrations at age 1, 2, and 3 years.
Adjusted for maternal age at delivery, race, BMI at 16 weeks gestation, household income, and gestational week at sample collection
TSH concentrations were ln-transformed. Thus, we exponentiated the beta coefficients to show the percent change in TSH concentration with a 10-fold increase in urinary triclosan concentrations [(eβ−1)×100]
Adjusted for maternal age at delivery, race, BMI at 16 weeks gestation, household income, and child sex
Adjusted for child race, household income, child BMI at age 3 years, and child age at 3 years.
We observed that average gestational urinary triclosan concentrations were inversely associated with cord serum TT4 and fT3 concentrations and positively associated with cord serum TSH concentrations (Table 2); however, the 95% confidence intervals (CIs) of these point estimates included the null value. The inverse associations between triclosan concentrations and TT4 were stronger for triclosan measures taken near the time of delivery (β: −0.3; 95% CI: −0.6, −0.0) than measures at 16 (β: −0.1; 95% CI: −0.4, 0.2) or 26 weeks (β: −0.2; 95% CI: −0.4, 0.1) (Figure 2, Supplemental Table 3); however, these associations were not statistically different from one another (visit x triclosan interaction term p-value=0.33) (Supplemental Table 4). The association between individual urinary triclosan measures and fT3 concentrations did not significantly vary according to the time of urine sample collection (visit x triclosan interaction term p-value=0.85) (Supplemental Table 4). The repeated triclosan concentrations were not associated with other cord serum thyroid hormone concentrations (Supplemental Table 4).
Figure 2.
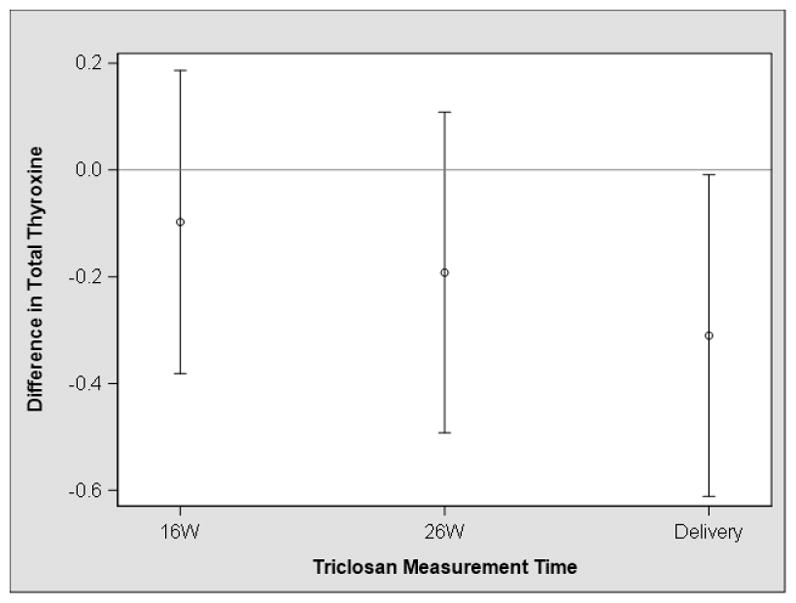
Adjusted difference in neonatal cord serum total thyroxine concentrations with a 10-fold increase in urinary triclosan concentrations at 16 and 26 weeks and near the time of deliverya,b
a- Adjusted for maternal age at delivery, race, BMI at 16 weeks gestation, household income, and child sex
b-Estimates are derived from a multiple informants model that simultaneously estimates the association between each urinary triclosan concentration and serum total thyroxine concentrations. Estimates were not significantly different from one another (visit x triclosan p-value=0.33).
Average gestational urinary triclosan concentrations were positively associated with TSH concentrations at age 3 years; however, the 95% CI of this estimate included the null value (Table 2). The associations of individual triclosan measures with TSH concentrations were not statistically different from one another (visit x triclosan interaction term p-value=0.90). Repeated triclosan measures during gestation or childhood were not associated with serum concentrations of other thyroid hormones at age 3 years (Supplemental Table 5). Average childhood urinary triclosan concentrations were positively associated with TT4 and fT4 concentrations at age 3 years (Table 2). The association of TT4 and fT4 concentrations at age 3 years with triclosan were strongest for triclosan measures collected at age 1 year (β TT4: 0.7; 95% CI: 0.3, 1.2; β fT4: 0.05; 95% CI: 0, 0.09) compared to triclosan concentrations at other time points (Figure 3, Supplemental Table 5), but the associations of repeated triclosan measures were only different from each other when examining triclosan and TT4 (visit x triclosan interaction term p-value=0.07) (Supplemental Table 5).
Figure 3.
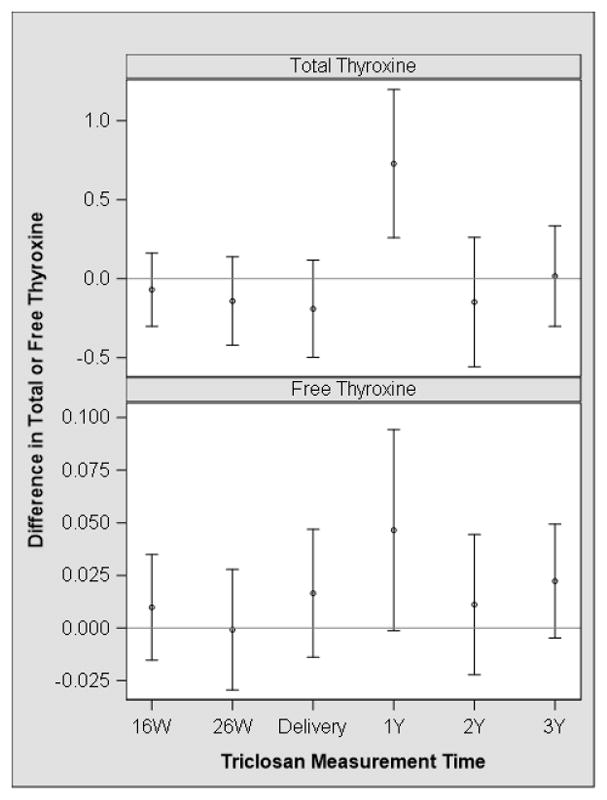
Adjusted difference in child serum total and free thyroxine concentrations at age 3 years with a 10-fold increase in urinary triclosan concentrations during pregnancy and early childhooda,b
a - Adjusted for child race, household income, child BMI at age 3 years, and child age at 3 years.
b-Estimates are derived from a multiple informants model that simultaneously estimates the association between each urinary triclosan concentration and serum thyroid hormone concentrations. Estimates were not significantly different from one another (visit x triclosan p-value=0.07 and 0.39 for total thyroxine and free thyroxine, respectively).
Secondary Analyses
We did not observe evidence that the association between urinary triclosan concentrations and thyroid hormones at delivery or age 3 years was modified by child sex, with one exception. The association between average gestational triclosan concentrations and cord serum TSH was positive in girls (56% increase in TSH per 10-fold increase in triclosan; 95% CI: 8, 125%), but null in boys (−19% decrease in TSH per 10-fold increase in triclosan; 95% CI: −46, 22) (child sex x triclosan interaction term p-value=0.02). Urinary iodine concentrations during pregnancy did not modify the association of gestational triclosan concentrations with maternal or neonatal thyroid hormone concentrations, with one exception. Among women with low iodine concentrations during pregnancy, triclosan was positively correlated with TSH concentrations at 16 weeks (67% increase; 95% CI: −20, 247), but inversely correlated with TSH among women with normal iodine concentrations (41% decrease; 95% CI: −62, 9) (iodine x triclosan interaction term p-value=0.02).
TPO antibody concentrations at 16 weeks gestation or delivery were not associated with gestational triclosan concentrations. Notably, we observed that each 10-fold increase in average childhood urinary triclosan concentrations were associated with 328% higher (95% CI: 18, 1,457) TPO antibody concentrations. These associations did not vary by visit (visit x triclosan interaction term p-value=0.15) (Supplemental Table 6).
Sensitivity Analyses
The associations of urinary triclosan concentrations around the time of delivery with TT4 did not appreciably change when we excluded neonates with high TPO antibody concentrations or NICU admission or when we adjusted for average gestational urinary BPA concentrations, urinary iodine concentrations, or delivery mode (Supplemental Table 7).
Discussion
We estimated the associations of urinary triclosan concentrations with thyroid hormone levels in a group of healthy pregnant women who gave birth to typically developing children with urinary triclosan concentrations similar to those observed in pregnant women and children from the United States (Calafat et al., 2008; Woodruff et al., 2011). In this cohort, we observed a few associations between urinary triclosan concentrations during pregnancy or early childhood and neonatal or child thyroid levels. Notably, urinary triclosan concentrations during pregnancy were inversely associated with serum TT4 concentrations in neonates at delivery. In particular, higher urinary triclosan concentrations near the time of delivery were more strongly associated with decreases in TT4 concentrations than triclosan concentrations at 16 or 26 weeks gestation. Urinary triclosan concentrations during pregnancy or childhood were generally not associated with most thyroid hormone concentrations at age 3 years. However, we did observe suggestive positive associations of gestational triclosan concentrations with TSH concentrations at age 3 years and childhood triclosan concentrations with TT4 and fT4 concentrations at age 3 years. We did not observe evidence that urinary triclosan concentrations during pregnancy were associated with maternal thyroid hormones at 16 weeks gestation.
This study adds to a small number of epidemiological studies examining the potential thyroid-toxicity of triclosan exposure. In a randomized trial of adults who had coronary heart disease (n=132), TSH, fT4, and fT3 concentrations did not change after five years of using triclosan-containing toothpaste vs. regular toothpaste (Cullinan et al., 2012). A small cross-over study of adults (n=12) did not observe any changes in T4 concentrations after 14 days of using a triclosan-containing toothpaste (Allmyr et al., 2009). In a nationally representative cross-sectional study of United States adolescents and adults (n=1,831), urinary triclosan concentrations (median~10 μg/g Cr) were associated with elevated TT3 concentrations in adolescents; triclosan concentrations (median=11 to 16 μg/g Cr) were not associated with thyroid hormone concentrations in adults (Koeppe et al., 2013). In a study of overweight or obese Belgian adults (n=194), urinary triclosan concentrations (median=1.5 ng/mL) were inversely associated with fT4 concentrations at baseline and 3 months after bariatric surgery or a weight loss program, with stronger associations in women compared to men (Geens et al., 2015). Finally, in a study of 398 Chinese women, urinary triclosan concentrations at ~39 weeks gestation (median=2.5 ng/mL) were inversely associated with maternal fT4 concentrations at 36–41 weeks gestation and cord serum fT3 concentrations (Wang et al., 2017). Both the present and prior study observed evidence that urinary triclosan concentrations around the time of delivery were inversely associated with neonatal thyroid hormone levels. In contrast to the inverse association between triclosan and fT4 among pregnant women in this prior study, we observed no association between triclosan and fT4 concentrations at 16 weeks gestation.
Differences in the results of our study and the prior one in pregnant women could arise for several reasons (Wang et al., 2017). First, we assessed triclosan exposure in both the 2nd and 3rd trimesters and again near the time of delivery, while the prior study assessed triclosan exposure in the late 3rd trimester. Second, average (median: 13 vs. 2.5 ng/mL) and maximum (maximum: 1,135 vs. 89 ng/mL) third trimester urinary triclosan concentrations were higher in this study compared to the study in China (Supplemental Table 2). Finally, we assessed thyroid function earlier in the pregnancy than the prior study. Given the dynamic state of maternal thyroid function during pregnancy, it is possible that the effects of triclosan on thyroid concentrations vary over the course of pregnancy (de Escobar et al., 2004). Additional studies with repeated measures of both triclosan exposure and thyroid function during pregnancy could address this hypothesis.
One prior cross-sectional study observed a positive correlation between urinary triclosan concentrations and triiodothyronine concentrations among adolescents (Koeppe et al., 2013). This is somewhat consistent with our results of a positive association of childhood triclosan concentrations with serum TT4 or fT4 concentrations at age 3 years, but neither this nor the prior cross-sectional study are consistent with experimental studies observing decreased thyroxine concentrations in triclosan-exposed juvenile rodents (Johnson et al., 2016). We also observed a suggestive positive association between gestational urinary triclosan concentrations and serum TSH concentrations at age 3 years. Additional studies examining early life triclosan exposure and child thyroid function with larger sample sizes are warranted given our notable findings.
At least nine experimental studies in rodents show that triclosan exposure can reduce circulating concentrations of T4 in pregnant, neonatal, juvenile, and adult mice or rats (Johnson et al., 2016; Louis et al., 2017). Several experimental studies in rodents and in vitro systems show that triclosan may decrease circulating T4 and T3 concentrations by increasing thyroid hormone clearance via hepatic catabolism. Paul et al. showed that triclosan caused reductions in serum TT4 and TT3 concentrations in adult rats and these decreases were accompanied by increased T4 glucuronidation and upregulation of Phase II enzymes in the liver that are involved in thyroid hormone catabolism (Paul et al. 2010). In a second study, Paul et al. demonstrated that triclosan caused reductions in maternal and neonatal serum TT4 concentrations and this was accompanied by changes in the activity of Phase II enzymes in the maternal liver that were consistent with a hepatic catabolism mechanism of action (Paul et al., 2012). Triclosan may have other effects on thyroid hormone metabolism, including sulfotransferase (Butt and Stapleton, 2013) or deiodinase inhibition (Butt et al., 2011; Shimizu et al., 2013), which may in turn affect the availability of iodine for thyroid hormone synthesis.
We used a novel statistical method to identify potential windows of susceptibility to triclosan exposure. Some of the associations between triclosan and thyroid hormones concentrations varied with respect to the timing of triclosan exposure assessment. We speculate that triclosan exposure during sensitive periods of development may influence the programming of thyroid hormone homeostasis. Prior studies have observed that the hypothalamic-pituitary-thyroid axis undergoes rapid changes during both gestation and infancy (Fisher et al., 2000), and that the programming of this hormonal axis is modulated by numerous factors, including genetics, endogenous hormones, and xenobiotics (Hoermann et al., 2015). One limitation of our statistical method was that it required us to make a large number of comparisons and it is possible that some results are spurious.
The present study has several strengths and limitations. First, we prospectively measured urinary triclosan concentration up to three times during pregnancy and at delivery, and up to three additional times during early childhood. Having repeated urinary triclosan concentrations allowed us to estimate average gestational and childhood exposure, while also identifying periods of heightened susceptibility. However there is moderate within-person variation of urinary triclosan concentrations during pregnancy and early childhood (Stacy et al., 2017), which can result in triclosan exposure misclassification. If this misclassification is non-differential with respect to thyroid hormone concentrations, then our associations may be attenuated towards the null. Second, we examined thyroid hormone concentrations at three periods of life when thyroid hormones play an important role in neurodevelopment (Zoeller and Rovet, 2004). Gestational T4 reductions are associated with deficits in visual processing, visuomotor abilities, and motor skills, while postnatal reductions are associated with deficits in language, fine motor skills, attention, and memory (Rovet et al., 1992; Rovet and Hepworth, 2001a; Rovet and Hepworth, 2001b; Song et al., 2001). Given that both gestational and childhood thyroid hormones are necessary for different aspects of neurodevelopment, there is the potential for triclosan exposure at different periods of life to impact specific features of neurobehavior. Nevertheless, our sample size for the analysis of thyroid hormone concentrations at age 3 years was small and we lacked statistical power to detect subtle associations at this time. In addition, our modest sample size prevented us from determining whether urinary triclosan concentrations were associated with clinically significant changes in thyroid function. Finally, while we examined whether maternal iodine status during pregnancy modified the associations between triclosan and thyroid hormones, we were not able to examine whether other known thyroid stressors (e.g., perchlorate exposure) modify associations between triclosan and thyroid hormone concentrations (Steinmaus et al., 2016). Moreover, we were not able to examine if childhood iodine status modified or confounded the association between triclosan and thyroid hormone concentrations.
This study contributes to the body of literature examining the potential thyroid toxicity of triclosan in humans. During pregnancy, urinary triclosan concentrations were not associated with thyroid hormone concentrations. However, urinary triclosan concentrations during pregnancy, particularly around the time of delivery, were associated with reduced thyroxine levels among newborns in this cohort. Triclosan concentrations in childhood, particularly those around age 1 year, were associated with increased thyroxine concentrations in children at age 3 years. Given that thyroid hormones play a critical role in neurodevelopment, additional studies are needed to determine if triclosan exposure is associated with adverse neurodevelopmental outcomes in children and if neonatal thyroid function mediates these associations.
Supplementary Material
Highlights.
We measured triclosan levels 3 times during gestation and 3 times during childhood
We measured thyroid function during pregnancy and at delivery and age 3 years
Triclosan levels during pregnancy were not associated with maternal thyroid levels
Triclosan levels at delivery were associated with reduced neonatal thyroxine levels
Triclosan levels at age 1 year were associated with higher child thyroxine levels
Acknowledgments
This work was supported by grants from the Environmental Protection Agency grant (RD – 83544201) and National Institute of Environmental Health Sciences (R01 ES024381, R01 ES020349, P01 ES11261, R01 ES014575, and P01 ES022832).
Footnotes
Competing Financial Interests: The authors have no competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alexander EK, Pearce EN, Brent GA, Brown RS, Chen H, Dosiou C, Grobman WA, Laurberg P, Lazarus JH, Mandel SJ, Peeters RP, Sullivan S. 2017 Guidelines of the American Thyroid Association for the Diagnosis and Management of Thyroid Disease During Pregnancy and the Postpartum. Thyroid: official journal of the American Thyroid Association. 2017;27:315–389. doi: 10.1089/thy.2016.0457. [DOI] [PubMed] [Google Scholar]
- Allmyr M, Adolfsson-Erici M, McLachlan MS, Sandborgh-Englund G. Triclosan in plasma and milk from Swedish nursing mothers and their exposure via personal care products. The Science of the total environment. 2006;372:87–93. doi: 10.1016/j.scitotenv.2006.08.007. [DOI] [PubMed] [Google Scholar]
- Allmyr M, Panagiotidis G, Sparve E, Diczfalusy U, Sandborgh-Englund G. Human exposure to triclosan via toothpaste does not change CYP3A4 activity or plasma concentrations of thyroid hormones. Basic & clinical pharmacology & toxicology. 2009;105:339–344. doi: 10.1111/j.1742-7843.2009.00455.x. [DOI] [PubMed] [Google Scholar]
- Bernert JT, Jacob P, Holiday DB, Benowitz NL, Sosnoff CS, Doig MV, Feyerabend C, Aldous KM, Sharifi M, Kellogg MD, Langman LJ. Interlaboratory comparability of serum cotinine measurements at smoker and nonsmoker concentration levels: a round-robin study. Nicotine Tob Res. 2009;11:1458–1466. doi: 10.1093/ntr/ntp161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun JM, Kalloo G, Chen A, Dietrich KN, Liddy-Hicks S, Morgan S, Xu Y, Yolton K, Lanphear BP. Cohort Profile: The Health Outcomes and Measures of the Environment (HOME) study. International journal of epidemiology. 2016;46:24. doi: 10.1093/ije/dyw006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butt CM, Stapleton HM. Inhibition of thyroid hormone sulfotransferase activity by brominated flame retardants and halogenated phenolics. Chemical research in toxicology. 2013;26:1692–1702. doi: 10.1021/tx400342k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butt CM, Wang D, Stapleton HM. Halogenated phenolic contaminants inhibit the in vitro activity of the thyroid-regulating deiodinases in human liver. Toxicol Sci. 2011;124:339–347. doi: 10.1093/toxsci/kfr117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calafat AM, Ye X, Wong LY, Reidy JA, Needham LL. Urinary concentrations of triclosan in the U.S. population: 2003–2004. Environmental health perspectives. 2008;116:303–307. doi: 10.1289/ehp.10768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell KL, Maxwell CB, Makhmudov A, Pino S, Braverman LE, Jones RL, Hollowell JG. Use of inductively coupled plasma mass spectrometry to measure urinary iodine in NHANES 2000: comparison with previous method. Clin Chem. 2003;49:1019–1021. doi: 10.1373/49.6.1019. [DOI] [PubMed] [Google Scholar]
- Casas L, Fernandez MF, Llop S, Guxens M, Ballester F, Olea N, Irurzun MB, Rodriguez LS, Riano I, Tardon A, Vrijheid M, Calafat AM, Sunyer J. Urinary concentrations of phthalates and phenols in a population of Spanish pregnant women and children. Environment international. 2011;37:858–866. doi: 10.1016/j.envint.2011.02.012. [DOI] [PubMed] [Google Scholar]
- Chevrier J. Invited commentary: Maternal plasma polybrominated diphenyl ethers and thyroid hormones--challenges and opportunities. American journal of epidemiology. 2013;178:714–719. doi: 10.1093/aje/kwt138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevrier J, Gunier RB, Bradman A, Holland NT, Calafat AM, Eskenazi B, Harley KG. Maternal urinary bisphenol a during pregnancy and maternal and neonatal thyroid function in the CHAMACOS study. Environmental health perspectives. 2013;121:138–144. doi: 10.1289/ehp.1205092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullinan MP, Palmer JE, Carle AD, West MJ, Seymour GJ. Long term use of triclosan toothpaste and thyroid function. The Science of the total environment. 2012;416:75–79. doi: 10.1016/j.scitotenv.2011.11.063. [DOI] [PubMed] [Google Scholar]
- Dann AB, Hontela A. Triclosan: environmental exposure, toxicity and mechanisms of action. J Appl Toxicol. 2011;31:285–311. doi: 10.1002/jat.1660. [DOI] [PubMed] [Google Scholar]
- de Escobar GM, Obregon MJ, del Rey FE. Maternal thyroid hormones early in pregnancy and fetal brain development. Best practice & research. Clinical endocrinology & metabolism. 2004;18:225–248. doi: 10.1016/j.beem.2004.03.012. [DOI] [PubMed] [Google Scholar]
- Desquilbet L, Mariotti F. Dose-response analyses using restricted cubic spline functions in public health research. Statistics in medicine. 2010 doi: 10.1002/sim.3841. [DOI] [PubMed] [Google Scholar]
- Fisher DA, Nelson JC, Carlton EI, Wilcox RB. Maturation of human hypothalamic-pituitary-thyroid function and control. Thyroid: official journal of the American Thyroid Association. 2000;10:229–234. doi: 10.1089/thy.2000.10.229. [DOI] [PubMed] [Google Scholar]
- Geens T, Dirtu AC, Dirinck E, Malarvannan G, Van Gaal L, Jorens PG, Covaci A. Daily intake of bisphenol A and triclosan and their association with anthropometric data, thyroid hormones and weight loss in overweight and obese individuals. Environment international. 2015;76C:98–105. doi: 10.1016/j.envint.2014.12.003. [DOI] [PubMed] [Google Scholar]
- Ghassabian A, Bongers-Schokking JJ, Henrichs J, Jaddoe VW, Visser TJ, Visser W, de Muinck Keizer-Schrama SM, Hooijkaas H, Steegers EA, Hofman A, Verhulst FC, van der Ende J, de Rijke YB, Tiemeier H. Maternal thyroid function during pregnancy and behavioral problems in the offspring: the generation R study. Pediatric research. 2011;69:454–459. doi: 10.1203/PDR.0b013e3182125b0c. [DOI] [PubMed] [Google Scholar]
- Henrichs J, Ghassabian A, Peeters RP, Tiemeier H. Maternal hypothyroxinemia and effects on cognitive functioning in childhood: how and why? Clinical endocrinology. 2013;79:152–162. doi: 10.1111/cen.12227. [DOI] [PubMed] [Google Scholar]
- Hoermann R, Midgley JE, Larisch R, Dietrich JW. Homeostatic Control of the Thyroid-Pituitary Axis: Perspectives for Diagnosis and Treatment. Frontiers in endocrinology. 2015;6:177. doi: 10.3389/fendo.2015.00177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson PI, Koustas E, Vesterinen HM, Sutton P, Atchley DS, Kim AN, Campbell M, Donald JM, Sen S, Bero L, Zeise L, Woodruff TJ. Application of the Navigation Guide systematic review methodology to the evidence for developmental and reproductive toxicity of triclosan. Environment international. 2016 doi: 10.1016/j.envint.2016.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koeppe ES, Ferguson KK, Colacino JA, Meeker JD. Relationship between urinary triclosan and paraben concentrations and serum thyroid measures in NHANES 2007–2008. The Science of the total environment. 2013;445–446:299–305. doi: 10.1016/j.scitotenv.2012.12.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Ying GG, Zhao JL, Chen ZF, Lai HJ, Su HC. 4-Nonylphenol, bisphenol-A and triclosan levels in human urine of children and students in China, and the effects of drinking these bottled materials on the levels. Environment international. 2013;52:81–86. doi: 10.1016/j.envint.2011.03.026. [DOI] [PubMed] [Google Scholar]
- Louis GW, Hallinger DR, Braxton MJ, Kamel A, Stoker TE. Effects of chronic exposure to triclosan on reproductive and thyroid endpoints in the adult Wistar female rat. Journal of toxicology and environmental health. 2017:1–14. doi: 10.1080/15287394.2017.1287029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manglik AK, Chatterjee N, Ghosh G. Umbilical cord blood TSH levels in term neonates: a screening tool for congenital hypothyroidism. Indian Pediatr. 2005;42:1029–1032. [PubMed] [Google Scholar]
- Paul KB, Hedge JM, Bansal R, Zoeller RT, Peter R, DeVito MJ, Crofton KM. Developmental triclosan exposure decreases maternal, fetal, and early neonatal thyroxine: a dynamic and kinetic evaluation of a putative mode-of-action. Toxicology. 2012;300:31–45. doi: 10.1016/j.tox.2012.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul KB, Hedge JM, DeVito MJ, Crofton KM. Short-term exposure to triclosan decreases thyroxine in vivo via upregulation of hepatic catabolism in Young Long-Evans rats. Toxicological sciences: an official journal of the Society of Toxicology. 2010;113:367–379. doi: 10.1093/toxsci/kfp271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodricks JV, Swenberg JA, Borzelleca JF, Maronpot RR, Shipp AM. Triclosan: a critical review of the experimental data and development of margins of safety for consumer products. Critical reviews in toxicology. 2010;40:422–484. doi: 10.3109/10408441003667514. [DOI] [PubMed] [Google Scholar]
- Romano ME, Webster GM, Vuong AM, Thomas Zoeller R, Chen A, Hoofnagle AN, Calafat AM, Karagas MR, Yolton K, Lanphear BP, Braun JM. Gestational urinary bisphenol A and maternal and newborn thyroid hormone concentrations: The HOME Study. Environmental research. 2015;138:453–460. doi: 10.1016/j.envres.2015.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rovet JF, Ehrlich RM, Sorbara DL. Neurodevelopment in infants and preschool children with congenital hypothyroidism: etiological and treatment factors affecting outcome. Journal of pediatric psychology. 1992;17:187–213. doi: 10.1093/jpepsy/17.2.187. [DOI] [PubMed] [Google Scholar]
- Rovet JF, Hepworth S. Attention problems in adolescents with congenital hypothyroidism: a multicomponential analysis. J Int Neuropsychol Soc. 2001a;7:734–744. doi: 10.1017/s135561770176609x. [DOI] [PubMed] [Google Scholar]
- Rovet JF, Hepworth SL. Dissociating attention deficits in children with ADHD and congenital hypothyroidism using multiple CPTs. Journal of child psychology and psychiatry, and allied disciplines. 2001b;42:1049–1056. doi: 10.1111/1469-7610.00804. [DOI] [PubMed] [Google Scholar]
- Sanchez BN, Hu H, Litman HJ, Tellez-Rojo MM. Statistical methods to study timing of vulnerability with sparsely sampled data on environmental toxicants. Environmental health perspectives. 2011;119:409–415. doi: 10.1289/ehp.1002453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandborgh-Englund G, Adolfsson-Erici M, Odham G, Ekstrand J. Pharmacokinetics of triclosan following oral ingestion in humans. Journal of toxicology and environmental health. 2006;69:1861–1873. doi: 10.1080/15287390600631706. [DOI] [PubMed] [Google Scholar]
- Shimizu R, Yamaguchi M, Uramaru N, Kuroki H, Ohta S, Kitamura S, Sugihara K. Structure-activity relationships of 44 halogenated compounds for iodotyrosine deiodinase-inhibitory activity. Toxicology. 2013;314:22–29. doi: 10.1016/j.tox.2013.08.017. [DOI] [PubMed] [Google Scholar]
- Song SI, Daneman D, Rovet J. The influence of etiology and treatment factors on intellectual outcome in congenital hypothyroidism. J Dev Behav Pediatr. 2001;22:376–384. doi: 10.1097/00004703-200112000-00005. [DOI] [PubMed] [Google Scholar]
- Stacy SL, Eliot M, Etzel T, Papandonatos G, Calafat AM, Chen A, Hauser R, Lanphear BP, Sathyanarayana S, Ye X, Yolton K, Braun JM. Patterns, Variability, and Predictors of Urinary Triclosan Concentrations during Pregnancy and Childhood. Environmental science & technology. 2017;51:6404–6413. doi: 10.1021/acs.est.7b00325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmaus C, Pearl M, Kharrazi M, Blount BC, Miller MD, Pearce EN, Valentin-Blasini L, DeLorenze G, Hoofnagle AN, Liaw J. Thyroid Hormones and Moderate Exposure to Perchlorate during Pregnancy in Women in Southern California. Environmental health perspectives. 2016;124:861–867. doi: 10.1289/ehp.1409614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Ouyang F, Feng L, Wang X, Liu Z, Zhang J. Maternal Urinary Triclosan Concentration in Relation to Maternal and Neonatal Thyroid Hormone Levels: A Prospective Study. Environmental health perspectives. 2017;125:067017. doi: 10.1289/EHP500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff MS, Teitelbaum SL, Pinney SM, Windham G, Liao L, Biro F, Kushi LH, Erdmann C, Hiatt RA, Rybak ME, Calafat AM, Cancer B. Investigation of Relationships between Urinary Biomarkers of Phytoestrogens, Phthalates, and Phenols and Pubertal Stages in Girls. Environmental health perspectives. 2010;118:1039–1046. doi: 10.1289/ehp.0901690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff TJ, Zota AR, Schwartz JM. Environmental chemicals in pregnant women in the United States: NHANES 2003–2004. Environmental health perspectives. 2011;119:878–885. doi: 10.1289/ehp.1002727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye X, Bishop AM, Needham LL, Calafat AM. Automated on-line column-switching HPLC-MS/MS method with peak focusing for measuring parabens, triclosan, and other environmental phenols in human milk. Anal Chim Acta. 2008;622:150–156. doi: 10.1016/j.aca.2008.05.068. [DOI] [PubMed] [Google Scholar]
- Ye X, Zhou X, Hennings R, Kramer J, Calafat AM. Potential external contamination with bisphenol A and other ubiquitous organic environmental chemicals during biomonitoring analysis: an elusive laboratory challenge. Environmental health perspectives. 2013;121:283–286. doi: 10.1289/ehp.1206093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoeller RT, Rovet J. Timing of thyroid hormone action in the developing brain: clinical observations and experimental findings. Journal of neuroendocrinology. 2004;16:809–818. doi: 10.1111/j.1365-2826.2004.01243.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.