Summary
Background
In adult urologic oncology the use of robotics has become commonplace; in pediatric urology it is rare. Herein, we describe a collaboration between an adult and a pediatric urologist performing robotic surgery for children and young adults with suspicious or cancerous genitourinary (GU) lesions.
Objectives
To evaluate clinical and oncologic outcomes in children and young adults undergoing robotic surgery for suspicious or cancerous lesions of the GU tract; to describe our collaborative model between an adult and pediatric surgeon at a free-standing children’s hospital.
Design
We retrospectively reviewed all robotic cases performed at our institution from 2014 to 2016 for patients with a GU malignancy or a suspicious mass. The surgeries were performed by a pediatric urologist with robotic experience and a fellowship-trained MIS adult urologist specializing in oncology. Perioperative and oncologic outcomes were recorded.
Results
A total of eight robotic cases were performed: four partial nephrectomies (PN) with retroperitoneal lymph node dissection (LND) (OT 269–338 min, EBL 5–300 mL, LOS 3–6 days), one adrenalectomy with LND (6.4 cm mass; OT 172 min, EBL 5 mL, LOS 3 days), one nephrectomy with pericaval LND (9.8 cm mass; 234 min, EBL 25 mL, LOS 3 days), and two retroperitoneal LNDs (OT 572 and 508 min, EBL 250 and 100, LOS 3 and 4 days). Patient weights ranged from 14 to 79 kg (mean 53.4 kg). There were no major complications (Clavien 3–5). Pathology results for PN included papillary RCC (AJCC pT1aNx) and two cases of segmental cystic renal dysplasia with nephrogenic rests. Bilateral template RPLNDs yielded paratesticular rhabdomyosarcoma (43 nodes; COG low risk group II stage I) and mixed non-seminomatous germ cell tumor (74 nodes; COG stage III). The nephrectomy yielded an undifferentiated sarcoma, low grade; the adrenalectomy favorable-type ganglioneuroma.
Discussion
In pediatrics, urologic oncology cases are often managed with open surgery. Our series demonstrates the feasibility of using the robotic approach in carefully selected cases. In doing so, the patient benefits from a minimally invasive surgery, while the surgeon benefits from robotic surgical dexterity. We seamlessly advanced these new techniques through a step-wise collaboration between an adult urologist who routinely performs robotic oncology procedures and a pediatric urologist experienced in robotics for benign conditions.
Conclusion
In this small series, we safely and effectively adapted adult robotic techniques for genitourinary oncology cases in children and young adults.
Keywords: Robotics, Oncology, Partial nephrectomy, Retroperitoneal lymph node dissection, Adrenalectomy, Radical nephrectomy
Introduction
Since the first report of a robotic radical prostatectomy in 2001 [1], the use of robot-assistance in urologic oncology has expanded. In fact, for radical prostatectomy and partial nephrectomy (PN) the number of annual robotic cases has surpassed that of the open approach [2,3]. The use of robotic surgery for cystoprostatectomy has also been gaining momentum [4], with several institutions reporting experience with intracorporeal ileal conduit and neobladder creation [5,6]. Most recently, the use of the robotic platform for retroperitoneal lymph node dissection has been shown to be safe and feasible in carefully selected adult patients [7].
Despite the widespread dissemination of robotic surgery in adult urologic oncology, the use of robotics in pediatric urologic oncology has been slow to progress. In the current literature, there are only four case reports of robotic techniques for cancers with pathology similar to adults in adolescent patients, namely renal cell carcinoma [10] and two non-seminomatous germ cell tumors [11], and one report of robotic nephrectomy being used safely for Wilms’ tumor in an adolescent patient [12]. The reasons for this are multifactorial: 1) genitourinary (GU) malignancies in pediatric patients are rare, making it difficult to amass case volume, 2) when they do occur, many are too large or complex for minimally invasive techniques, 3) the current management strategies for pediatric GU cancers are largely successful, therefore the incentive to test new management approaches is limited, and 4) a majority of pediatric GU oncology cases are managed by general surgeons [8,9] who generally have lower rates of robotic utilization than urologists.
At our institution, we collaborated with a fellowship-trained minimally invasive (MIS) urologist with 10 years of attending-surgeon experience with adult urologic oncology cases. This collaboration led to the successful transfer of surgical techniques for our adolescent patients, as well as the thoughtful adaptation of the adult techniques for our smaller children. Herein we present a series of eight cases of suspicious and malignant masses managed with robotic surgery in children and young adults.
Materials and methods
Patient population
Robotic cases performed at Boston Children’s Hospital between 2014 and 2016 were retrospectively reviewed. Patients with a preoperative diagnosis of a malignancy or suspicion for malignancy were included. Prior to surgery, each patient was also seen by a medical oncologist. Robotic surgery was then chosen via a multidisciplinary, patient-centered approach.
Partial nephrectomy
Four patients were referred for management of suspicious small renal masses. Median age was 12.5 years (range 3–26) with a median weight of 45 kg (range 14–79). Nephrometry scores ranged between 5 and 9. Table 1 provides the individual preoperative characteristics.
Table 1.
Preoperative characteristics of eight pediatric and young adult patients referred for excision of suspicious or cancerous lesions.
| Age (years) | Wt (kg) | Location of mass | Size of mass (cm) | Nephrometry score | Preoperative diagnosis |
|---|---|---|---|---|---|
| 26 | 57 | Renal | 3.5 | 8A | Suspicious mass |
| 13 | 79 | Renal | 3.2 | 9A | Suspicious mass |
| 3 | 14 | Renal | 2.1 | 5A | Suspicious mass |
| 12 | 33 | Renal | 1.5 | 8A | Suspicious mass |
| 17 | 75 | Retroperitoneal | 2.0 | – | Mixed NSGCT RP mass w LN |
| 16 | 78 | Retroperitoneal | na | – | Paratesticular RMS |
| 7 | 31 | Adrenal | 6.4 | – | Suspicious mass Elevated catecholamines |
| 19 | 60 | Renal | 9.8 | Suspicious mass, RP LN |
Wt = weight; NSGCT = non-seminomatous germ cell tumor; RP = retroperitoneum; LN = lymphadenopathy; RMS = rhabdomyosarcoma.
Retroperitoneal lymph node dissection
Two patients were referred for RPLND. The first was a 17-year-old male (75 kg) with a history of a mixed non-seminomatous germ cell tumor (NSGCT) status post orchiectomy with an enlarging aortocaval lymph node on surveillance imaging (Fig. 1). Pre-orchiectomy tumor markers were elevated (AFP 76, hCG 215, and LDH 248), but normalized following orchiectomy despite increasing lymphadenopathy. The second patient was a 16-year-old male (78 kg) with a history of paratesticular rhabdomyosarcoma with lymph node invasion status post orchiectomy.
Figure 1.
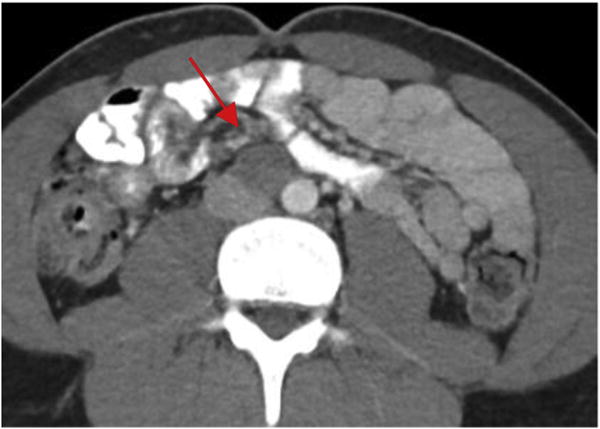
Enlarging aortocaval lymph node in a 17-year-old male with a history of mixed NSGCT managed initially with orchiectomy and surveillance.
Adrenalectomy and nephrectomy
A 7-year-old boy (31 kg) was referred for excision of a 6.4-cm adrenal mass (Fig. 2A) with elevated urine dopamine (dopamine 2690 mcg/gCr) but otherwise normal catecholamines (norepinephrine 120 mcg/gCr, and epinephrine 25 mcg/gCr). His blood pressures were within normal range for his age. A MIBG scan showed focal enhancement of the adrenal mass with no evidence of metastases.
Figure 2.
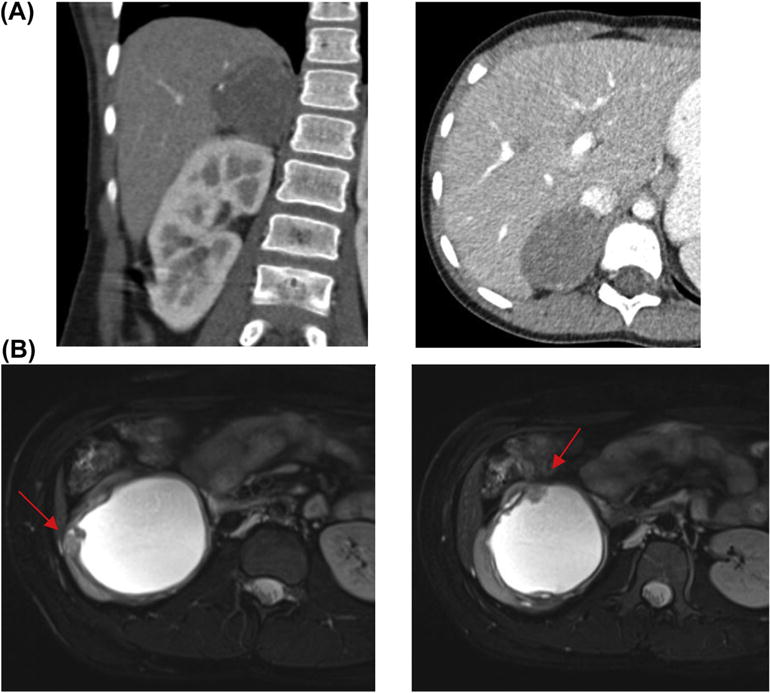
(A) Coronal and cross-sectional CT imaging with contrast of an adrenal mass in a 7-year-old boy with elevated urine catecholamines. (B) Cross-sectional T2 MRI imaging demonstrating suspicious cyst wall abnormalities in a 19-year-old girl found to have unclassified spindle cell carcinoma.
A 19-year-old girl (60 kg) was referred with a 9.8-cm cystic renal mass (Fig. 2B) She initially presented to an outside institution with pyelonephritis and was thought to have a large renal abscess. This was drained and the fluid sent for analysis which showed small round blue cells concerning for malignancy. She subsequently underwent a renal biopsy with the results concerning for a Wilms’ tumor variant.
Operative technique
At the start of the collaboration, pertinent surgical techniques, equipment, and video footage of adult cases were reviewed. Subsequently, adult robotic cases (PN and RPLND) were observed by the pediatric urology team. Prior to each pediatric case, both urologists were involved in patient-centered surgical planning. The pediatric cases were performed by RL and proctored by AW, who obtained surgical privileges at our institution and served as the bedside assistant. Real time adjustments were made during the case to optimize positioning, access, and surgical technique. Insufflation pressures of 8 mmHg were used for pre-pubertal patients, as well as microbipolar cautery and mini-lap sponges.
Four partial nephrectomies were performed. The port configuration included two 8-mm robotic working ports, a 12-mm camera port, one 12-mm assistant port, and a 5-mm assistant port. An additional 5-mm retractor port was used for right-sided procedures requiring liver retraction. The port placement was based on patient size and was adjusted for our smaller patients through general medialization of the port configuration (Fig. 3). A complete list of adaptations for our patients weighing 14 and 33 kg is presented in Table 2. Selective arterial clamping of segmental or polar vessels was performed when feasible. Closure of the nephrectomy bed was accomplished with a running 3–0 V-loc suture followed by early vascular unclamping. Arterial bleeding vessels were then closed using focal figure of eight sutures again using 3–0 V-loc sutures. This was repeated after dropping pneumoperitoneum. No hemostatic agents were used. A sliding clip renorrhaphy was then used to reapproximate the renal cortex with interrupted 0-VICRYL sutures [13]. A perihilar/aortocaval lymph node dissection was performed for the benefit of staging if pathology yielded a Wilms’ tumor. One nephrectomy with hilar lymph node dissection was performed. Port placement was similar to PN. The renal artery and vein were ligated using separate vascular endo-GIA stapler loads.
Figure 3.
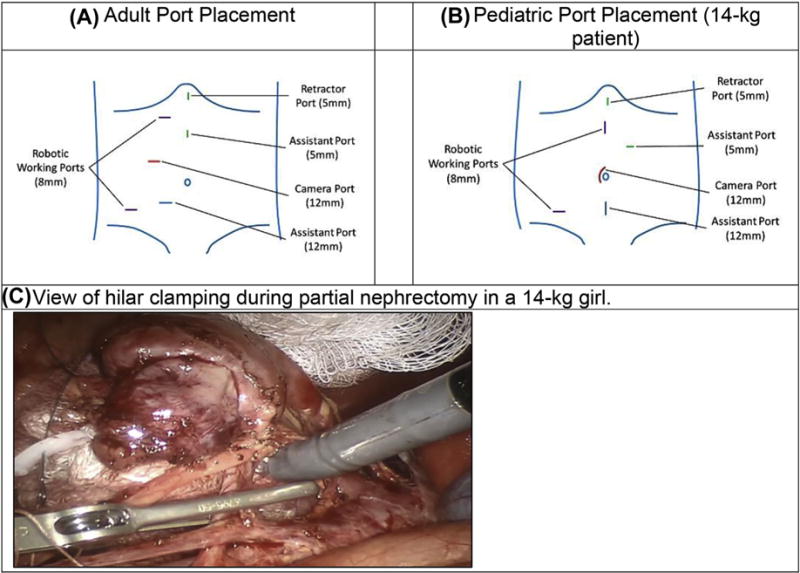
Adult to pediatric port placement for right robotic partial nephrectomy. (A) Adult port placement. (B) Pediatric port placement (14-kg patient). (C) View of hilar clamping during partial nephrectomy in a 14-kg girl.
Table 2.
Step-wise details of adaptations made for two children (14 and 33 kg) undergoing robotic partial nephrectomy for suspicious small renal masses.
| Adult (>70 kg) | Pediatric (≤33 kg) | |
|---|---|---|
| Preoperative | Clears, enema day prior | Clears, glycerin PR day prior |
| Positioning | Modified flank position | Modified flank position |
| Arm hugging a pillow | Arm at left side | |
| No axillary roll | No axillary roll | |
| Tape secured over arm, chest, hips, and legs | Tape secured over arm, chest, hips, and legs | |
| Ports | 12-mm camera port | 12-mm camera port |
| 8-mm left robotic port | 8-mm left robotic port | |
| 8-mm right robotic port | 8-mm right robotic port | |
| 12-mm assistant port | 12-mm assistant porta | |
| 5-mm assistant port | 5-mm assistant port | |
| (5-mm port for subxiphoid liver retractor) | (5-mm port for subxiphoid liver retractor) | |
| Insufflation | 15 mmHg for port placement | 12 mmHg for port placement |
| 15 mmHg for procedure | 8 mmHg for procedure | |
| 20 mmHg for tumor resection | 15 mmHg for tumor resection | |
| Instruments | Fenestrated bipolar (settings 30) | Microbipolar forceps (settings 15) |
| Monopolar scissors (settings 30, 30) | Monopolar scissors (settings 15,15) | |
| Laparoscopic suction/irrigator | Laparoscopic suction/irrigator | |
| Locking graspers (Hunter) | Locking graspers (Hunter) | |
| Clamping | 12.5 mg mannitol (optional) | Weight based mannitol (0.4 mg/kg; optional) |
| Artery only on left, artery and vein on right | Artery only on left, artery and vein on right | |
| Early unclamping after tumor bed closed | Early unclamping after tumor bed closed | |
| Tumor resection | Demarcate w ultrasound guidance | Demarcate w ultrasound guidance |
| 4 × 4 radio-tagged sponge pre-placedb | 2 × 2 radio-tagged sponge pre-placedb | |
| Cold scissors | Cold scissors | |
| Closure of tumor bed | 12 inch running 3–0 V-LOC stitch | 6 inch running 3–0 V-LOC stitch |
| Sliding clip renorrhaphy | 5 inch 0-VICRYL w Weck clip and knot at end | 4 inch 0-VICRYL w Weck clip and knot at end |
| Interrupted capsule to capsule stitches | Interrupted capsule to capsule stitches | |
| Weck clips to suture exiting capsule | Weck clips to suture exiting capsule |
12-mm port required for passage of ultrasound probe.
Preplaced if needed for hemostasis, may also be used to position kidney optimally for resection and suturing.
Two nerve-sparing bilateral template RPLNDs were performed. Port placement was generally similar to robotic kidney surgery with ports shifted slightly medially to allow for improved access to the great vessels. The right-sided dissection was performed first, followed by re-positioning of the patient for access to the contralateral side. The left and right colon were mobilized; the right colon beyond the cecum and the duodenum was Kocherized to provide complete exposure. Borders of the dissections included the ureters laterally, the iliac vessels inferiorly, and the renal hilum superiorly. The great vessels were carefully distracted to expose the spinous ligament and ensure complete dissection; this was facilitated by use of the fourth robotic arm. A portion of the dissection of the spermatic cord into the deep pelvis, down to the inguinal ring, was completed with pure laparoscopy given port site limitations. A series of both metal and Hem-o-lok clips were used to isolate the lymphatic tissue. Post-ganglionic sympathetic nerve fibers were identified and spared. Postoperatively, both patients were maintained on a low-fat diet for 2 weeks for prevention of chylous ascites.
Finally, one robotic adrenalectomy was performed. The same number and type of ports as the PN were used. However, the adult configuration was modified for our pediatric patient; this included widening the distance between the two robotic ports and generally shifting the port configuration towards the contralateral hip (Fig. 4). The inferior vena cava and renal vein were first identified; subsequent cephalad dissection led to identification of the adrenal vein. This was isolated and controlled using two Weck Hem-o-lok clips. Once divided, the remaining stellate adrenal arteries were managed with the microbipolar robotic forceps. The mass and adrenal gland were ultimately freed and removed en bloc.
Figure 4.
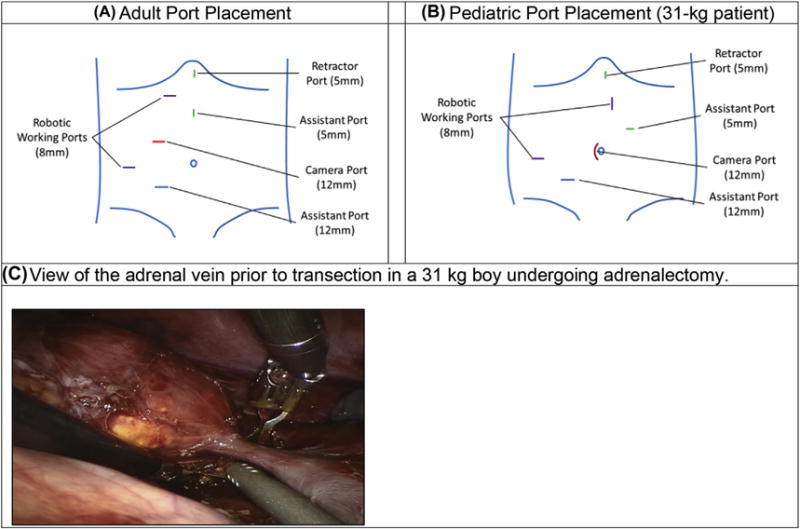
Adult to pediatric port placement for right robotic adrenalectomy. (A) Adult port placement. (B) Pediatric port placement (31-kg patient). (C) View of the adrenal vein prior to transection in a 31-kg boy undergoing adrenalectomy.
Results
Eight robotic cases were performed successfully: four partial nephrectomies, two bilateral nerve-sparing RPLND, one adrenalectomy, and one radical nephrectomy. Individual perioperative outcomes, including operative time (OT), warm ischemia time (WIT), estimated blood loss (EBL), and length of stay (LOS) are summarized in Table 3. Median operative time (OT) for PN was 277 min (median WIT 22 min) and 540 min for RPLND. There were no Clavien grade II–V complications. One PN patient developed a fever on POD0–1 associated with atelectasis that resolved with pulmonary toilette, while another PN patient was seen postoperatively in the emergency room for fevers and hives that were managed conservatively. There were no readmissions.
Table 3.
Perioperative outcomes for robotic partial nephrectomy, RPLND, adrenalectomy, and radical nephrectomy performed in eight children and young adults.
| Procedure | OT, min | WIT, min | EBL, mL | LOS, days |
|---|---|---|---|---|
| PN (57 kg, 8A) | 281 | 26 | 5 | 4 |
| PN (79 kg, 9A) | 338 | 32 | 300 | 4 |
| PN (14 kg, 5A) | 272 | 14 | 5 | 3 |
| PN (33 kg, 8A) | 269 | 18 | 50 | 6 |
| Bilateral NS-RPLND | 572 | – | 250 | 3 |
| Bilateral NS-RPLND | 508 | – | 100 | 4 |
| Adrenalectomy | 172 | – | 5 | 3 |
| Radical nephrectomy | 234 | – | 25 | 3 |
OT = operative time; WIT = warm ischemia time; EBL = estimated blood loss; LOS = length of stay; PN = partial nephrectomy; NS-RPLND = nerve-sparing retroperitoneal lymph node dissection.
Oncologic outcomes, including pathologic diagnosis, lymph node yield, and margin status are reported in Table 4. Follow-up information was available for all eight patients. The median follow-up time for all patients was 7 months. Both RPLND patients went on to further systemic therapy based on nodal involvement. At 3-month follow-up both patients reported normal erections with an ejaculation; one of the two reported return of antegrade ejaculation at 1 year. At the time, one patient had just completed additional chemotherapy, while the other was actively undergoing chemotherapy (includes agents associated with neuropathy) and radiation to the retroperitoneum. There was no evidence of recurrence for the patients with the ganglioneuroma, spindle cell sarcoma, or papillary RCC at 1, 1, and 2-year follow up imaging, respectively.
Table 4.
Oncologic outcomes for robotic partial nephrectomy, RPLND, adrenalectomy, and radical nephrectomy performed in eight children and young adults.
| Procedure | Pathology
|
||
|---|---|---|---|
| Diagnosis | Nodes | Margins | |
| Partial nephrectomy | Papillary RCC (pT1a) | 0 | Negative |
| Partial nephrectomy | Segmental cystic dysplasia | – | Negative |
| Partial nephrectomy | Benign heterologous tissue with nephrogenic rests | – | Negative |
| Partial nephrectomy | Segmental cystic dysplasia | 7 | Negative |
| Bilateral NS-RPLND | Embryonal NSGCT | 74 | – |
| Bilateral NS-RPLND | Rhabdomyosarcoma | 43 | – |
| Adrenalectomy | Ganglioneuroma (2A) | – | Positive |
| Radical nephrectomy | Unclassified spindle cell sarcoma | 4 | Negative |
RCC = renal cell carcinoma; NS-RPLND = nerve-sparing retroperitoneal lymph node dissection.
Discussion
The boundaries of what can be done with robotic surgery in pediatric urology are still expanding. We present a case series of suspicious and cancerous lesions in children and young adults that were successfully removed with robotic surgery. Among the eight cases performed, three were in very young children weighing between 14 and 33 kg. Perioperative outcomes were favorable with regard to blood loss and complications, while oncologic outcomes were promising regarding surgical margins, nodal counts, and recurrence-free follow-up. These encouraging results reflect a careful and systematic collaboration between an adult and pediatric urologist with extensive minimally invasive experience. By leveraging the unique robotic skill sets within our field, pediatric urologists can offer a safe and effective minimally invasive approach for patients with complicated GU lesions of childhood.
Among our set of cases, the robotic platform was markedly advantageous during partial nephrectomy, as it allowed free suturing of the nephrectomy bed, an extended lymphadenectomy, and the patient-level benefits of minimally invasive surgery. The timing of our experience parallels an increase in the use of open PN in pediatric patients with organ-confined renal masses. The Children’s Oncology Group (COG) protocol AREN0534 uses PN for patients with Wilms’ tumors and a solitary kidney, bilateral tumors, or predisposing tumor syndromes [15]. In these select cases, PN has been associated with equivalent overall and relapse-free survival to that of radical nephrectomy [16–18]. Currently, PN is under consideration for patients with node negative stage I tumors <550 g [19]. Theoretically, neoadjuvant chemotherapy could further expand the number of patients eligible for PN; however, this would represent a major shift in current treatment strategies for Wilms’ tumor in North America. In the case of pediatric RCC, the successful oncologic and renal function outcomes in the adult population strongly support PN for amenable renal masses regardless of age [14]. This emerging niche for partial nephrectomy presents an opportunity for pediatric urologists to usher in a robotic approach.
Another advantage of PN over nephrectomy is renal preservation [20]. This is especially important in children, as well as any patient who requires future systemic nephrotoxic therapy. We recapitulated renal preservation techniques used in adult RPN, including methods to decrease WIT such as early unclamping of the renal hilum and segmental vessel clamping. Additional methods of renal preservation, including tumor enucleation or “off-clamp” PN, have been demonstrated to be safe in adults and may also be possible in select pediatric cases.
Using the robotic approach for PN in children may provide the additional advantage of decreased EBL, shorter LOS, and reduced postoperative pain. In this series, we had minimal blood loss and a median LOS comparable with the adult literature [13]. Our median operative time of 277 min was longer than more recent reports for adult patients (140–233 min), but was similar to that from adult studies from 2006 to 2008 performed during a period of adoption [13]. We therefore expect operative times to improve with surgeon and institutional experience. Although we did not measure postoperative pain in this series, prior work comparing MIS and open PN for duplication anomalies has demonstrated better pain control among patients undergoing the MIS procedure [21,22]. In the case of our PN patients with benign disease, both the renal preservation and decreased surgical morbidity associated with the robotic approach were particularly salient.
Robotic RPLND was described in 2006 [23], and has since gained acceptance as an alternative to open RPLND [24]. In our two cases, we had short LOS, low EBL, considerable nodal yields, and no complications. Although RPLND may also be performed with pure laparoscopy, we found that the additional dexterity facilitated dissection around the great vessels. Our median operative time of 540 min was substantial compared with the adult literature (150–329 min) [24]; however, we performed bilateral re-sections in both cases. In addition, Cost et al. reported a similar OT (527 min) for a post-chemotherapy, bilateral RPLND in a 15-year-old male [11]. In regards to ejaculatory function, one patient has regained antegrade ejaculation, whereas the other has not. It is difficult to discern from a case series whether or not the robotic approach was associated with this outcome; however, the sympathetic chain and nerves were well-visualized in each case and meticulous dissection was performed. Other factors that may have influenced ejaculatory function include postoperative neuropathy related to chemotherapy, retroperitoneal radiation, and bulky lymphadenopathy near the inferior mesenteric artery. Both patients underwent preoperative fertility preservation prior to treatment.
Both pure laparoscopy and robot-assisted laparoscopy can be used safely and effectively for nephrectomy and adrenalectomy in adults. In our cases, we found that using the robotic approach allowed for careful manipulation and resection of two particularly large masses. In the case of the nephrectomy, the robotic dexterity and magnification also facilitated pericaval node dissection which was an advantage reported by Cost et al. in their case report of a pediatric robotic nephrectomy [12]. In regards to our adrenalectomy specimen, gross pathology demonstrated the mass abutting a thin rim of pseudocapsule, which is common for ganglioneuromas [25]. The positive margin of this benign tumor reported microscopically thus corresponded to a close resection rather than residual disease at the site of resection. There have been no signs of local recurrence on postoperative surveillance imaging.
The primary limitations of this study include the small number of procedures performed, a short median follow-up, and the lack of open comparison groups. However, the purpose of this proof-of-concept case series is to describe outcomes related to a new surgical technique, as well as a model for successful collaboration between an adult and pediatric urologist at a free-standing children’s hospital. Rigorous evaluations of these techniques will be feasible as uptake of the robotic approach expands.
Conclusion
Urologists have unique training in robotics, which can be applied in pediatric patients with GU oncology conditions. Using a robotic approach provides the benefits of minimally invasive surgery and considerable surgical dexterity not seen in laparoscopy. Our findings reflect a period of early adoption for this application of robotic surgery and collaboration with an experienced adult robotic surgeon was crucial. Operative times are expected to improve with experience. Notwithstanding, these procedures can be performed safely in children with comparable EBL, LOS, and complication rates to procedures performed in the adult population. Immediate oncologic outcomes appear to be favorable; however, long-term outcomes such as renal function, potency, recurrence, and survival must be further investigated. To advance robotic techniques in pediatric GU oncology, patients must be carefully selected and surgeries performed seamlessly. Thoughtful collaboration between an adult and a pediatric urologist with an oncologic focus is one way this can be achieved.
Figure.
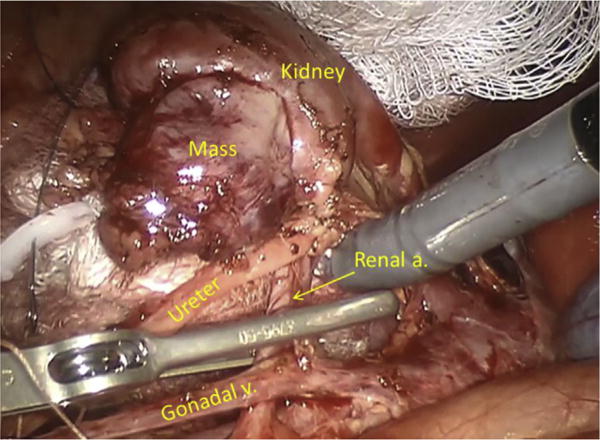
View of renal hilum during partial nephrectomy in a 14-kg girl.
Acknowledgments
Funding
Dr. Varda is supported by NICHD grant number T32HD075727. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Agency for Healthcare Research and Quality or the National Institutes of Health.
Footnotes
Conflicts of interest
None.
References
- 1.Abbou CC, Hoznek A, Salomon L, Olsson LE, Lobontiu A, Saint F, et al. Laparoscopic radical prostatectomy with a remote controlled robot. J Urol. 2001;165:1964–6. doi: 10.1097/00005392-200106000-00027. [DOI] [PubMed] [Google Scholar]
- 2.Chang SL, Kibel AS, Brooks JD, Chung BI. The impact of robotic surgery on the surgical management of prostate cancer in the USA. BJU Int. 2015;115:929–36. doi: 10.1111/bju.12850. [DOI] [PubMed] [Google Scholar]
- 3.Sammon JD, Karakiewicz PI, Sun M, Ravi P, Ghani KR, Jeong W, et al. Robot-assisted vs. laparoscopic partial nephrectomy: utilization rates and perioperative outcomes. Int Braz J Urol. 2013;39:377–86. doi: 10.1590/S1677-5538.IBJU.2013.03.11. [DOI] [PubMed] [Google Scholar]
- 4.Leow JJ, Reese SW, Jiang W, Lipsitz SR, Bellmunt J, Trinh Q-D, et al. Propensity-matched comparison of morbidity and costs of open and robot-assisted radical cystectomies: a contemporary population-based analysis in the United States. Eur Urol. 2014;66:569–76. doi: 10.1016/j.eururo.2014.01.029. [DOI] [PubMed] [Google Scholar]
- 5.Chopra S, de Castro Abreu AL, Berger AK, Sehgal S, Gill I, Aron M, et al. Evolution of robot-assisted orthotopic ileal neobladder formation: a step-by-step update to the University of Southern California (USC) technique. BJU Int. 2017 Jan;119(1):185–91. doi: 10.1111/bju.13611. [DOI] [PubMed] [Google Scholar]
- 6.Kurpad R, Woods M, Pruthi R. Current status of robot-assisted radical cystectomy and intracorporeal urinary diversion. Curr Urol Rep. 2016;17:42. doi: 10.1007/s11934-016-0598-y. https://doi.org/10.1007/s11934-016-0598-y. [DOI] [PubMed] [Google Scholar]
- 7.Pearce SM, Golan S, Gorin MA, Luckenbaugh AN, Williams SB, Ward JF, et al. Safety and early oncologic effectiveness of primary robotic retroperitoneal lymph node dissection for nonseminomatous germ cell testicular cancer. Eur Urol. 2017;71:476–82. doi: 10.1016/j.eururo.2016.05.017. [DOI] [PubMed] [Google Scholar]
- 8.Shnorhavorian M, Routh JC, Kieran K, Ritchey ML. Patterns of performance of surgical oncology by North American pediatric urologists –a report from the Pediatric Urologic Oncology Working Group (PUOWG) of the Society for Pediatric Urology. BJU Int. 2016;197(5):1349–54. doi: 10.1016/j.juro.2016.12.011. [DOI] [PubMed] [Google Scholar]
- 9.Ross JH. The role of urologists in the care of children with cancer. Urol Oncol. 2016;34:9–12. doi: 10.1016/j.urolonc.2015.06.004. [DOI] [PubMed] [Google Scholar]
- 10.Cost NG, Geller JI, Defoor WR, Wagner LM, Noh PH. A robotic-assisted laparoscopic approach for pediatric renal cell carcinoma allows for both nephron-sparing surgery and extended lymph node dissection. J Pediatr Surg. 2012;47:1946–50. doi: 10.1016/j.jpedsurg.2012.08.017. [DOI] [PubMed] [Google Scholar]
- 11.Cost NG, DaJusta DG, Granberg CF, Cooksey RM, Laborde CE, Wickiser JE, et al. Robot-assisted laparoscopic retroperitoneal lymph node dissection in an adolescent population. J Endourol. 2012;26:635–40. doi: 10.1089/end.2011.0214. [DOI] [PubMed] [Google Scholar]
- 12.Cost NG, Liss ZJ, Bean CM, Geller JI, Minevich EA, Noh PH. Prechemotherapy robotic-assisted laparoscopic radical nephrectomy for an adolescent with Wilms tumor. J Pediatr Hematol Oncol. 2015;37:e125–7. doi: 10.1097/MPH.0000000000000193. [DOI] [PubMed] [Google Scholar]
- 13.Cha EK, Lee DJ, Del Pizzo JJ. Current status of robotic partial nephrectomy (RPN) BJU Int. 2011;108:935–41. doi: 10.1111/j.1464-410X.2011.10556.x. [DOI] [PubMed] [Google Scholar]
- 14.Schiavina R, Novara G, Borghesi M, Ficarra V, Ahlawat R, Moon DA, et al. PADUA and R.E.N.A.L. nephrometry scores correlate with perioperative outcomes of robot-assisted partial nephrectomy: analysis of the Vattikuti Global Quality Initiative in Robotic Urologic Surgery (GQI-RUS) database. BJU Int. 2017;119:456–63. doi: 10.1111/bju.13628. [DOI] [PubMed] [Google Scholar]
- 15.Berg R, Bierman EN, Van Noord M. Nephron-sparing surgery for Wilms tumor: a systematic review. Urol Oncol. 2016;34(1):24–32. doi: 10.1016/j.urolonc.2015.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang HH, Abern MR, Cost NG, Chu DI, Ross SS, Wiener JS, et al. Use of nephron sparing surgery and impact on survival in children with Wilms tumor: a SEER analysis. J Urol. 2014 Oct;192(4):1196–202. doi: 10.1016/j.juro.2014.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haecker FM, von Schweinitz D, Harms D, Buerger D, Graf N. Partial nephrectomy for unilateral Wilms tumor: results of study SIOP 93–01/GPOH. J Urol. 2003;170(3):939–42. doi: 10.1097/01.ju.0000073848.33092.c7. [discussion 943–4] [DOI] [PubMed] [Google Scholar]
- 18.Cost NG, Lubahn JD, Granberg CF, Schlomer BJ, Wickiser JE, Rakheja D, et al. Oncologic outcomes of partial versus radical nephrectomy for unilateral Wilms tumor. Pediatr Blood Cancer. 2012;58:898–904. doi: 10.1002/pbc.23240. [DOI] [PubMed] [Google Scholar]
- 19.Ferrer FA, Rosen N, Herbst K, Fernandez CV, Khanna G, Dome JS, et al. Image based feasibility of renal sparing surgery for very low risk unilateral Wilms tumors: a report from the Children’s Oncology Group. J Urol. 2013;190:1846–51. doi: 10.1016/j.juro.2013.05.060. [DOI] [PubMed] [Google Scholar]
- 20.Cost NG, Sawicz-Birkowska K, Kajbafzadeh A-M, Tourchi A, Parigi GB, Guillén G, et al. A comparison of renal function outcomes after nephron-sparing surgery and radical nephrectomy for nonsyndromic unilateral Wilms tumor. Urology. 2014;83:1388–93. doi: 10.1016/j.urology.2014.01.051. [DOI] [PubMed] [Google Scholar]
- 21.Lee RS, Retik AB, Borer JG, Diamond DA, Peters CA. Pediatric retroperitoneal laparoscopic partial nephrectomy: comparison with an age matched cohort of open surgery. J Urol. 2005;174:708–12. doi: 10.1097/01.ju.0000164748.00339.4c. [DOI] [PubMed] [Google Scholar]
- 22.Robinson BC, Snow BW, Cartwright PC, De Vries CR, Hamilton BD, Anderson JB. Comparison of laparoscopic versus open partial nephrectomy in a pediatric series. J Urol. 2003;169:638–40. doi: 10.1097/01.ju.0000040332.77090.d2. [DOI] [PubMed] [Google Scholar]
- 23.Davol P, Sumfest J, Rukstalis D. Robotic-assisted laparoscopic retroperitoneal lymph node dissection. Urology. 2006;67:199. doi: 10.1016/j.urology.2005.07.022. [DOI] [PubMed] [Google Scholar]
- 24.Chalfin HJ, Ludwig W, Pierorazio PM, Allaf ME. Robotic primary RPLND for stage I testicular cancer: a review of indications and outcomes. Curr Urol Rep. 2016;17:41. doi: 10.1007/s11934-016-0597-z. [DOI] [PubMed] [Google Scholar]
- 25.Shawa H, Elsayes KM, Javadi S, Morani A, Williams MD, Lee JE, et al. Adrenal ganglioneuroma: features and outcomes of 27 cases at a referral cancer centre. Clin Endocrinol. 2014;80:342–7. doi: 10.1111/cen.12320. [DOI] [PubMed] [Google Scholar]


