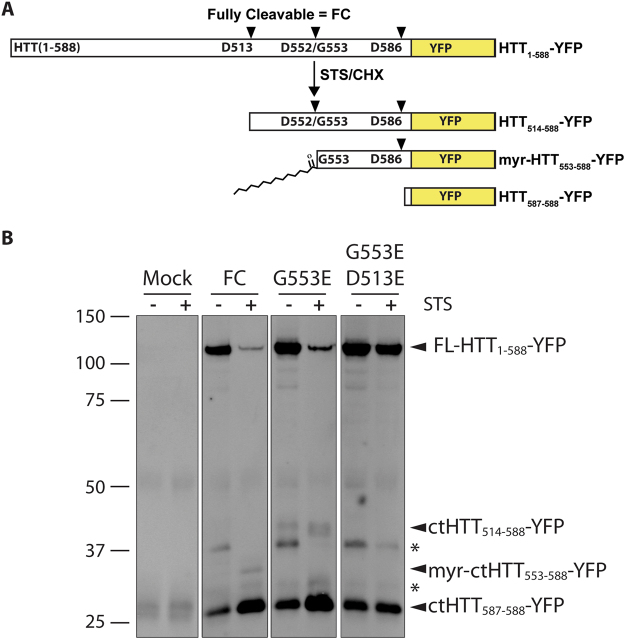
Figure 3.
The G553E substitution blocks post-translational myristoylation and alters proteolysis of wtHTT. (A) Schematic representation of proteolytic cleavage of WT fully cleavable (FC) HTT1–588-YFP. HTT is proteolysed by caspases at D586 and D552 and post-translationally myristoylated at G553. (B) HeLa cells were transfected with WT HTT1–588-YFP bearing the indicated point mutations. Proteolysis was induced with staurosporine (STS) and cycloheximide. In the presence of the G553E mutation, a higher molecular weight band was detected that was blocked when D513 was mutated to E, confirming the D513 caspase cleavage site. *Denotes unknown or non-specific bands. Image is a composite of lanes from the same Western blot.