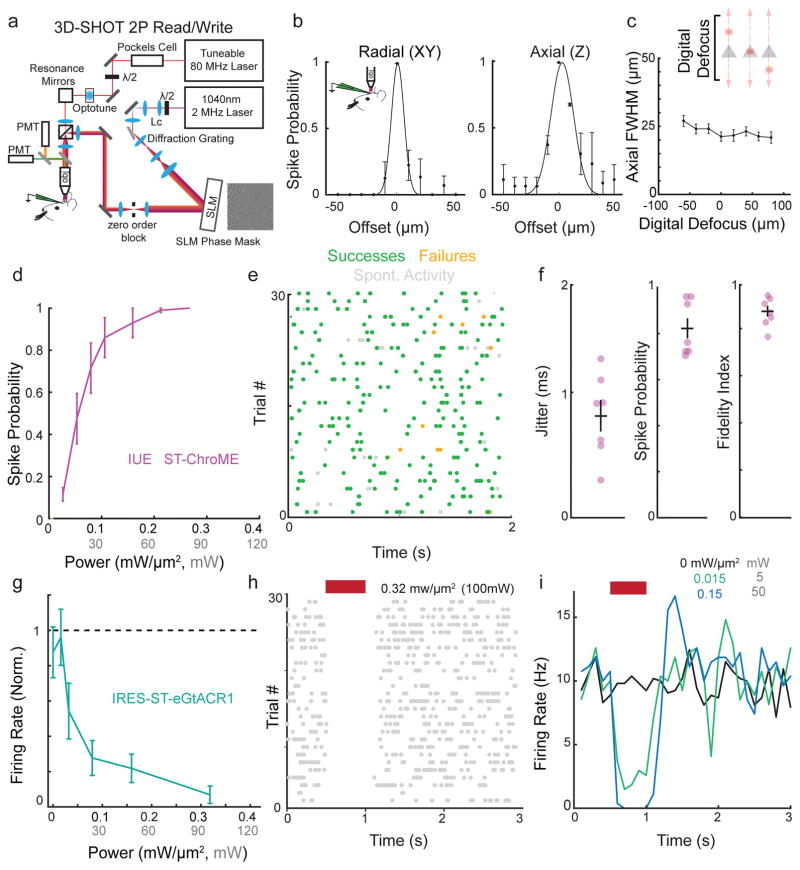
Figure 3. Creating and editing spatiotemporal neural activity in vivo.
a) Simplified schematic of light path allowing simultaneous 2P imaging and 3D-SHOT photostimulation.
b) Physiological point spread function (PPSF) of 3D-SHOT stimulation of neurons measured by in vivo loose patch. Left, spike probability for radial (XY) axis; right for axial (Z) axis (n=3 neurons).
c) In vivo recording of 3D-SHOT’s axial PPSF as a function of distance from the system’s zero order. PPSFs were measured as a function of depth by testing the spiking response to digital defocusing of the hologram while mechanically offsetting the objective varying distances from the focal plane (n=3 neurons).
d) Spike probability as a function of stimulation power in vivo for 1Hz stimulation in L2/3 pyramidal neurons expressing ST-ChroME-mRuby2 via IUE (n = 10 neurons).
e) Representative experiment showing in vivo Poisson stimulation of a L2/3 neuron expressing ST-ChroME.
f) Jitter, spike probability, and fidelity index score for Poisson stimulation of L2/3 neurons expressing ST-ChroME (n = 7 neurons).
g) Firing rate of neurons during stimulation normalized to pre-stimulation rate and measured through in vivo loose patch recordings from cells expressing IRES-ST-eGtACR1 (n=9 neurons).
h) Representative raster plot from a neuron suppressed with 500 ms stimulation.
i) Representative histogram of firing rate during IRES-ST-eGtACR1 suppression at several stimulation powers for the same neuron as in (h). Each line is the mean of 25+ stimulations binned at 100ms. All data indicate mean and s.e.m.