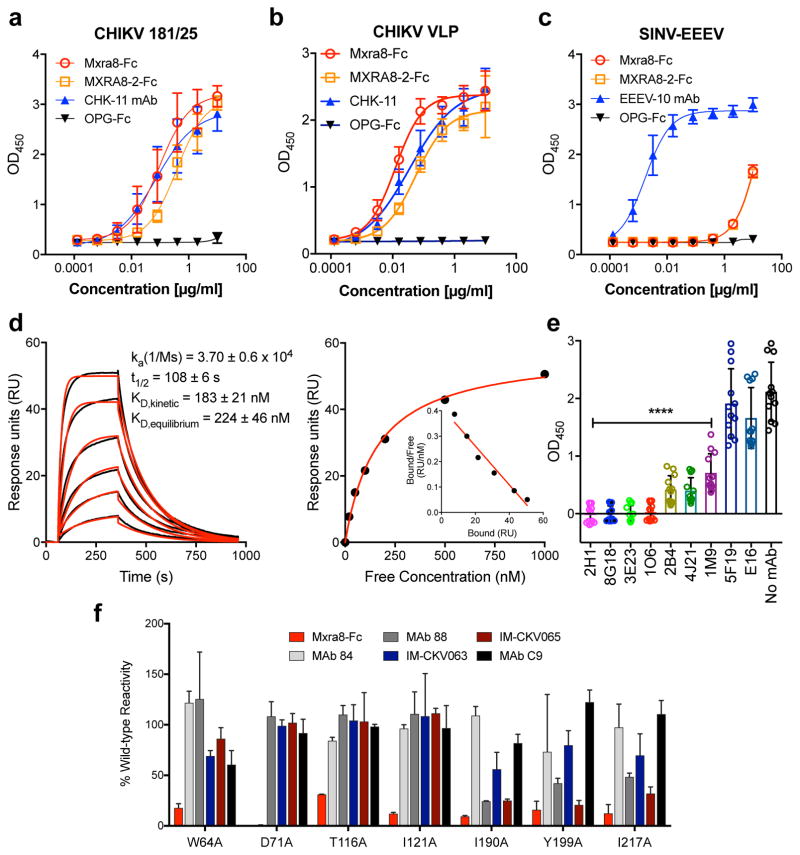
Figure 3. Direct binding of Mxra8 to CHIKV.
a–c. Purified CHIKV-181/25 (a), CHIKV VLPs (b), or chimeric EEEV virions (c) were captured with anti-CHIKV or -EEEV human mAbs. Mxra8-Fc, MXRA8-2-Fc, or OPG-Fc and positive controls mAbs (CHK-11 or EEEV-10) were added. Data are from two (b, c) or three (a) experiments (a, n = 8; b, n = 4; c, n = 6): mean ± SD. d. (Left) Sensograms of Mxra8 binding to CHIKV VLP. Experimental curves (black traces) were fit using a 1:1 binding model (red traces). (Right) Representative response curve for steady-state analysis, where binding is plotted versus Mxra8 concentration. Inset. Linear Scatchard plot (4 experiments; mean and standard error of the mean). e. Antibody blockade of Mxra8-Fc binding to CHIKV. Virus was incubated with indicated human mAbs against CHIKV, WNV (E16), or no mAb prior to the addition of Mxra8-Fc (4 experiments, n = 12; one-way ANOVA with Dunnett’s test, ****, P < 0.001, mean ± SD). f. Residues that result in loss of Mxra8-Fc binding to cell surface displayed CHIKV E2-E1. Residues are considered involved in the epitope if there is diminished binding without loss of protein integrity as judged by retention of interaction with mAbs (except for those previously mapped to a specific residue). Graphs are shown for four A domain (W64, D71, T116, and I121A) and three B domain (I190, Y199, and I217) alanine mutations (see also Supplementary Table 3). Data are from two experiments.