Abstract
Background and Purpose
Percutaneous transcatheter closure of patent foramen ovale (PFO closure) plus antiplatelet therapy has been shown to reduce the risk of recurrent stroke compared to medical therapy alone in carefully selected patients following cryptogenic stroke presumed to be from paradoxical embolism. Our objective was to determine the cost-effectiveness of PFO closure after cryptogenic stroke compared to conservative medical management from a United States healthcare payer perspective.
Methods
A decision analytic Markov model estimated the lifetime cost and outcomes associated with the additional benefit of PFO closure compared with medical management alone. Model inputs were obtained from published literature, national databases, and a meta-analysis of 5 published randomized clinical trials on PFO closure. Health outcomes were measured in quality-adjusted life years (QALY). Cost effectiveness used the incremental cost per QALY gained whereas the net monetary benefit assumed a willingness to pay of $150,000/QALY. One way and probabilistic sensitivity analyses estimated the uncertainty of model results.
Results
At 15 years, PFO closure compared to medical therapy alone improved QALY by 0.33 at a cost savings of $3,568, representing an incremental net monetary benefit of $52,761 (95% Interval −$8284 to $158,910). When the meta-analysis hazard ratio for stroke was increased to the 95% interval’s upper bound of 0.77, one-way sensitivity analyses suggested PFO closure’s cost effectiveness was $458,558 per additional QALY. Probabilistic sensitivity analysis suggested cost-effectiveness in 90% of simulation runs.
Conclusion
In conclusion, PFO closure for cryptogenic strokes in the right setting is cost-effective, producing benefit in QALYs gained and potential cost savings. However, patient selection remains vitally important as marginal declines in treatment effectiveness can dramatically affect cost effectiveness.
MeSH Terms: Stroke, Patent Foramen Ovale Closure, Embolic Stroke, Cost Benefit Analysis
Subject Terms: schemic Stroke, Embolism, Treatment, Cost-Effectiveness
INTRODUCTION
Cryptogenic strokes, or strokes with undetermined cause, represent 25 to 40% of all strokes and occur in higher frequencies among younger patients.1 While the prevalence of patent foramen ovale (PFO) in the general population is about 25%, the prevalence among patients with cryptogenic strokes is 40–60%.2, 3 This has led to the conclusion that paradoxical emboli through a PFO may be an important etiology missed in otherwise cryptogenic strokes. Previously, three randomized clinical trials, CLOSURE I (Evaluation of the STARFlex Septal Closure System in Patients with a Stroke and/or Transient Ischemic Attack due to Presumed Paradoxical Embolism through a PFO), RESPECT (Randomized Evaluation of Recurrent Stroke Comparing PFO Closure to Established Current Standard of Care Treatment), and PC (Percutaneous Closure of PFO in Cryptogenic Embolism) individually did not show a benefit of closure for secondary stroke prevention in the intention to treat (ITT) population.4–6 The patient level meta-analysis, however, did show benefit, particularly for use of the Amplatzer PFO Occluder, used in RESPECT and PC trials.7 Recently, two additional trials, REDUCE (GORE® HELEX® Septal Occluder / GORE® CARDIOFORM Septal Occluder and Antiplatelet Medical Management for Reduction of Recurrent Stroke or Imaging-Confirmed TIA in Patients With PFO) and CLOSE (PFO Closure or Anticoagulants versus Antiplatelet Therapy to Prevent Stroke Recurrence) and the extended follow up of RESPECT have all found a statistically significant benefit in the ITT population for PFO closure compared to medical management.8–10
Younger patients with strokes have the potential to accrue more costs over their lifetime both from disability and lost income. Hence, therapies that reduce stroke recurrences in this population are vitally important. Although multiple randomized trials have now demonstrated the efficacy of PFO closure, it is costly and associated with some complications in an otherwise younger, healthier stroke population. The objective of this study was to determine the cost-effectiveness of PFO closure after cryptogenic stroke compared to conservative medical management in the US from a healthcare payer perspective using a model based on the meta-analysis of the 5 randomized clinical trials, with a 15 year time horizon.
METHODS
Overview
The authors declare that all supporting data are available within the article. A previously published decision analytic model was adapted in Microsoft Excel to analyze the cost-effectiveness of PFO closure in addition to antiplatelet medication compared to medical therapy alone (i.e., anti-platelet, anti-coagulation, or both) for the secondary prevention of strokes.11 We combined outcome and procedural complication data from all 5 randomized clinical trials: CLOSURE I, RESPECT (the planned analysis), PC, REDUCE, and CLOSE. Institutional review was exempted because this study used only previously published data.
Model Structure
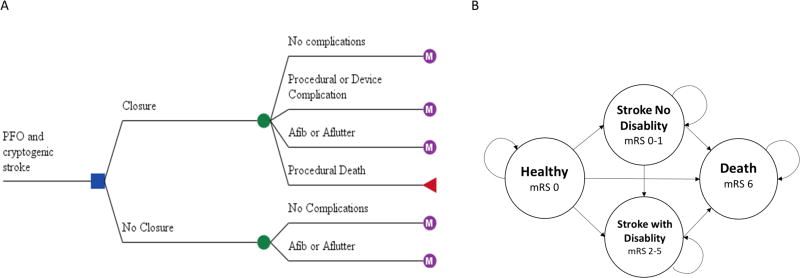
Patients with PFO enter the model after a stroke, transient ischemic attack (TIA), or systemic emboli. Overwhelmingly, the most common event was a cryptogenic stroke (93% of the patients across all 5 trials), but two earlier trials, CLOSURE I and PC, also included some patients with TIA and systemic emboli, respectively. All patients were assumed to be 45 years old at the time of index event, which was the mean age among patients in the first 3 trials (CLOSURE I, RESPECT, and PC), using patient level meta-analysis.7 Stroke disability is quantified using the modified Rankin Score (mRS), with mRS 0 indicating no symptoms, mRS 1 no significant disability, mRS 2 minor disability, mRS 3 moderate disability, mRS 4 moderate to severe disability, mRS 5 severe disability and mRS 6 indicating death. The decision tree analyzes the cost of a complication associated with PFO closure, including device and procedural complications, atrial fibrillation, and procedural death (figure 1A). It is possible to find incidental atrial fibrillation after randomization in the medical group, which would be unrelated to PFO closure.
Figure 1.
Model Structure. A, Decision analytic tree. Patient enters the model after a cryptogenic stroke and receives either medical therapy or patent foramen ovale (PFO) closure. B, Markov state transition model. At the end of each annual cycle the patient may remain in the healthy state, suffer a recurrent stroke and be independent or dependentor die. mRS indicates modified Rankin’s score, Afib is atrial fibrillation, and Aflutter is atrial flutter.
In our previous model assessing the efficacy of intra-arterial thrombectomy after acute stroke, we had broken down each mRS as a discrete Markov state.11 However, unlike interventional therapy, which ameliorates disability of strokes, we assume that PFO closure does not lessen the severity of subsequent strokes compared to non-closure. Since the distribution of disability in each arm is the same, we opted for a simpler Markov model consisting of four Markov states. All patients entered the Markov model, where they remained healthy, suffered a stroke and is independent (mRS 0–2), suffered a stroke and is dependent (mRS 3–5), or died (figure 1 B). Patients with dependent strokes cannot lose their disability, but can die, whereas, patients with non-dependent strokes may suffer another stroke and become dependent or die.
In the RESPECT trial, only 13% of recurrent strokes in those greater than 60 years old were cryptogenic, compared to 82% which were cryptogenic in subjects 60 years or younger.12 The mean age of patients entering this model is 45 years old, so we chose a time horizon of 15 years to avoid confounding of strokes due to other etiologies. The cycle length is 1 year. Death is the only absorbing state, after which patients were excluded from the model.
Input Parameters
Model input parameters were drawn from published literature, the meta-analysis of trials, and the National Inpatient Sample (Table 1). This study used only costs based on actual patient data in the US. Whenever possible, this study relied on assumptions used in prior peer-reviewed cost-effectiveness analysis models to enhance the validity of these findings and to maximize comparability to other stroke treatments.
Table 1.
Base-case values and plausible ranges of model inputs.
| Model Input | Base Case | Range | Reference |
|---|---|---|---|
| Probability: | |||
| Procedural complications | 4.1% | 3.0 – 5.6% | Meta-analysis |
| Atrial fibrillation or flutter | 4.0% | 2.3 – 6.9% | Meta-analysis |
| Procedural death | 0% | 0.001 – 0.5% | Meta-analysis |
| Recurrent stroke with closure | 0.5% | - | Meta-analysis |
| Recurrent stroke with dependence | 25.6% | 7 – 45%‖ | Meta-analysis |
| Death with recurrent stroke | 0% | 0.001 – 0.5% | Meta-analysis |
| Utility: | |||
| Penalty for procedural complications | −0.15 | −0.25 – 0 | Tengs 200013 |
| Penalty for atrial fibrillation | −0.05 | −0.1 – 0 | Tengs 200013 |
| Stroke, Independent (mRS 0–2) | 0.74 | 0.66 – 0.95 | Ganesalingam, 201514 |
| Stroke, Dependent (mRS 3–5) | 0.38 | 0.25 – 0.66 | Ganesalingam, 201514 |
| Death | 0 | - | |
| Hazard Ratio: | |||
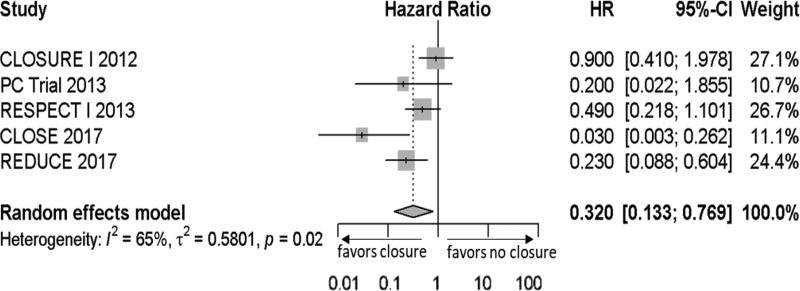
| Recurrent stroke with no closure | 0.32 | 0.13–0.77 | Meta-analysis (figure 2) |
| Death with stroke, independent | 1.5 | 1 – 2 | Slot, 200815 |
| Death with stroke, dependent | 2.67 | 1.5 – 3.8 | Eriksson, 200816 |
| Cost: | |||
| Acute stroke, mild | $19,112 | $9556 –34,225 | Brinjikji 201217 |
| Acute stroke, moderate/severe | $27,249 | $13,625 – 54,499 | Brinjikji 201217 |
| Acute stroke, death | $32,853 | $16,426–65,705 | Brinjikji 201217 |
| PFO Closure | $16,623 | $8,312–33,246 | Supplement table I |
| Closure complication | $12,593 | $6,296–25,186 | Supplement table II |
| Atrial fibrillation or flutter | $6,448 | $3,224–$12,897 | Shah 201618 |
| Annual posthospitalization (mRS 0–2) | $12,084 | $6042–24,169 | Shireman 201719 |
| Annual posthospitalization (mRS 3–5) | $46,043 | $23,021–92,085 | Shireman 201719 |
Probabilities
Due to the heterogeneity of the medical arm (anticoagulation, antiplatelet, or both), we calculated the annualized stroke rate in the more homogenous PFO closure arm (closure plus antiplatelet in all trials) and applied the inverse hazard ratio of closure from the meta-analysis, assuming random effects (figure 2), to get the annualized stroke rate of the medical arm. Annualized rates of stroke in both arms were converted into an annual probability for the model. The probability of procedural complications and atrial fibrillation were taken from the meta-analysis. The most common complications (n>2) were major bleeding, cardiac thrombus, cardiac tamponade, stroke, and device dislocation. There were no deaths directly due to PFO closure and no deaths from recurrent strokes, however these variables were included to test their potential influence on cost effectiveness and a small sensitivity range was introduced. Two of the five trials, CLOSE and RESPECT, reported functional outcomes after recurrent strokes. In CLOSE, 1 of 14 (7.1%) recurrent strokes had a mRS >2.8 In RESPECT, 9 of 25 (36%) recurrent stroke had a mRS >2.12 Altogether the risk of stroke with dependence (mRS >2) was 25.6%. However, due to the relatively small sample, we introduced an even wider sensitivity range between 7 to 45% to account for this uncertainty (which is larger than would be expected using a beta distribution with alpha of 10 and beta of 29). We assumed the same probability of disabling recurrent strokes in both the treatment and medical arms.
Figure 2.
Forest plot of hazard ratio or treatment effect size. Hazard ratios of recurrent stroke for patent foramen ovale (PFO) closure by each randomized clinical trial is listed. Mean represented by gray box (size is proportional to the weight) and black lines on either end represents 95% confidence intervals. Summary measure (dotted line through gray diamond) and associated confidence interval (lateral tips of diamond) assumes random effects model. Right of the solid vertical line is no benefit from treatment, left represents benefit. HR indicates hazard ratio, CI is confidence interval.
Cost
The cost of acute stroke hospitalization was taken from previous literature using US nationwide estimates for patients less than 65 years old broken down by discharge disposition, a surrogate for stroke severity.17 Due to the variability of private health insurance reimbursement in the US, the cost of PFO closure was tabulated from standard Medicare reimbursement rates.20 Charges for PFO closure include 2 echocardiograms,2 outpatient visits and percutaneous transcatheter closure as an outpatient (see Supplement table I). Cost of closure complications were estimated based on the proportion of a complication multiplied by its associated national average cost identified by the International Classification of Disease (ICD)-9 code (see Supplement table II).21 The additional cost of atrial fibrillation or flutter assumes the cost of warfarin treatment for 15 years, or the time horizon of the model.18 Annual post-hospitalization costs based on mRS were taken from a previously published stroke model based on a cohort of 958 acute stroke patients in US centers with strokes between 2010 and 2014.19 All future costs were discounted by 3% per year, consistent with current guidelines.22 All costs before 2017 were inflated to 2017 US Dollars (USD) according to the medical care component of the Consumer Price Index.
Outcome Assessment
Utility for each Markov state, including healthy (mRS 0), stroke without dependence (mRS 0–2), stroke with dependence (mRS 3–5) and death, ranged from 0 to 1, where a utility of 1 represents no loss of utility and 0 is death or no utility at all. Utility values were taken from previously validated stroke models using the same health states.14 Respective utilities at the end of each cycle were summed over the time horizon to get QALYs. Future QALYs were discounted by 3% per year, in accordance with current guidelines.22
The incremental cost and QALY between PFO closure and medical treatment were assessed. The incremental cost effectiveness ratio (ICER) was obtained by dividing the cost difference by the difference in QALY. This is commonly interpreted as the cost per an additional QALY gained. A treatment producing benefit in QALY as well as cost saving is referred to as the dominant strategy. Given that negative ICERs are hard to interpret, we also calculated the incremental net monetary benefit (INMB) of treatment given by the equation: (difference in QALY) X WTP – (difference in cost).23 The interpretation of INMB is intuitive, where a positive value indicates cost-effective and negative if not. Since patients enter the model at 45 years old, during their prime working years, we set the higher willingness to pay (WTP) threshold of $150,000 per QALY, based on current recommendations for the US.24
Sensitivity Analysis
To estimate the uncertainty of each input parameter on model results, we used a deterministic one-way sensitivity analysis. All input parameters, including transitional probabilities, health utilities, hazard ratios and costs, were varied by a pre-specified sensitivity range. Values derived by the meta-analysis, including probabilities of procedural complications, probability of atrial fibrillation or flutter in both arms, and hazard ratio of non-closure, were varied by their 95% confidence intervals. Death hazard ratios after stroke and health utilities were varied based on consensus of an expert panel, and all cost variables were varied by a magnitude of 0.5 and two.
A probabilistic sensitivity analysis using Monte Carlo simulation was performed, in which all input parameters were varied simultaneously with mean defined as the base case and range defined as two standard deviations from the mean in either direction. Transitional probabilities and health state utilities all varied by a beta distribution. Hazard ratios varied by log normal distribution. All costs were varied using a gamma distribution. The analysis was run 10,000 times to capture stability in the results. Uncertainty was represented using a scatter plot. Since the time horizon can change depending on age of the patient at the time of treatment, we also represented the probability of cost effectiveness in a surface plot with varying time horizon and WTP.
RESULTS
Base-Case Analysis
Over a 15-year time horizon, PFO closure yielded 12.4 QALYs at a cost of $29,282 and medical therapy yielded 12.0 QALYs at a cost of $32,850, suggesting that PFO closure resulted in a gain of 0.33 QALYs at cost savings of $3,568. Thus, PFO closure dominates medical therapy and is associated with on average 120 additional days of perfect health over a 15 year time horizon compared to medical therapy, translating to a positive incremental net monetary benefit of $52,761. In order to understand how long it would take to reach cost effectiveness, we calculated the ICER for the base case varying the time horizon from 5–25 years (Supplemental figure I). By seven years, PFO closure satisfies the WTP threshold at a cost of $149,624 per QALY gained and at 14 years, PFO closure becomes the dominant strategy.
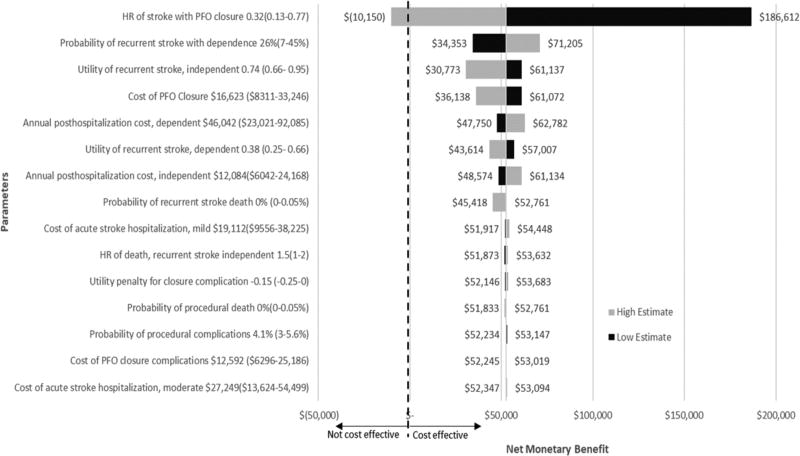
One-Way Sensitivity Analysis
With the exception of the hazard ratio of recurrent stroke, variation across all other variables still resulted in acceptable limits of cost effectiveness, defined as WTP of $150,000 (figure 3). The model was most sensitive to the hazard ratio of stroke with closure or the magnitude of treatment benefit. With the high hazard ratio estimate of 0.77, indicating less benefit from closure, the INMB became negative, costing $10,150; with ICER of $458,558 per additional QALY. On the other extreme, with a hazard ratio of 0.13, the INMB increases to $155,849, producing an improvement of 0.98 in QALY and $44,944 in cost savings.
Figure 3.
One-way sensitivity analysis: effect of parameter variation on the net monetary benefit using willingness to pay of $150,000 per QALY. Dark bars represents the upper input bound, light bars represents the lower input bound. Right of the dashed line represents cost effectiveness, left of the dashed line is not cost effective for PFO. Baseline net monetary benefit was $52,761. PFO indicates patent foramen ovale; and HR, hazard ratio.
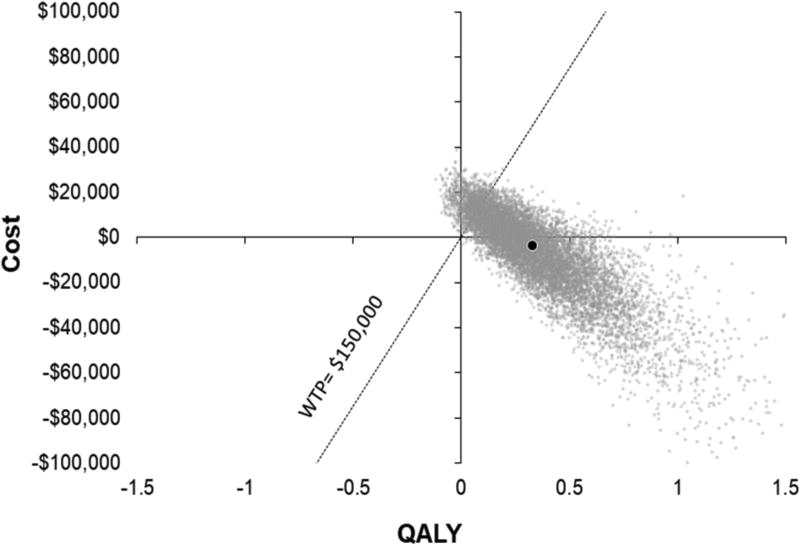
Multiway Probabilistic Sensitivity Analysis
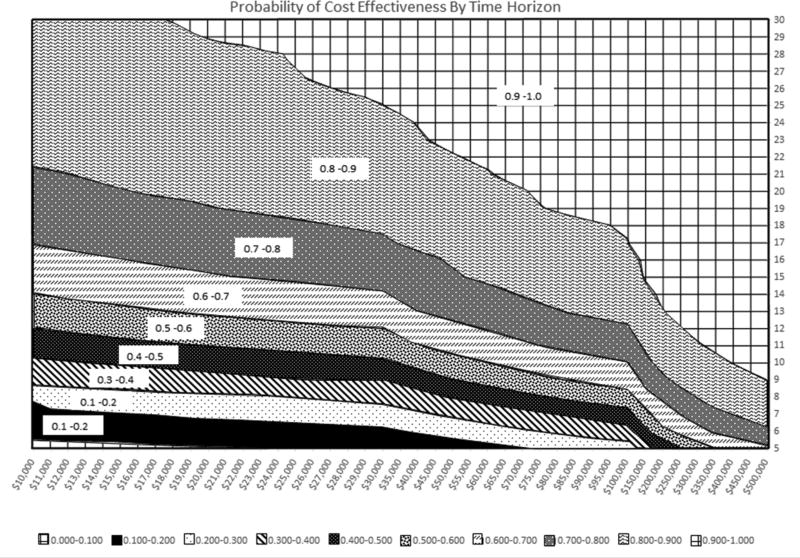
The results of the multiway sensitivity analysis are shown in figure 4. In 57.8% of the simulations run, PFO closure was the dominant strategy with a benefit in QALYs and cost savings. In 97.7% of the simulations run, PFO closure was beneficial, with more QALYs than medical therapy. Finally, in 90.0% of the simulations, PFO closure was cost effective at a WTP threshold of $150,000/QALY. The 95% interval for INMB at a WTP of $150,000 ranged from −$8284 to $158,910. The surface plot in figure 5 demonstrates the probability of cost effectiveness based on the WTP and the time horizon or years after PFO closure. For example, at WTP of $50,000 we would attain a probability of > 90% cost effectiveness by 25 years. As expected, the probability of cost effectiveness increased with longer time and increased threshold for WTP.
Figure 4.
Multiway probabilistic sensitivity analysis. Incremental cost-effectiveness scatter plot of patients treated with PFO closure compared with medical therapy alone. Dotted line represents an incremental cost per quality adjusted life year (QALY) of $150,000. Points to the right of the dotted line are considered cost effective. Each gray dot represents a simulation run. The black dot represents the base-case result of −$3568 and 0.33 QALYs. WTP indicates willingness to pay.
Figure 5.
Surface plot. Probability of cost-effectiveness or percentage of the 10,000 simulation at a particular year (time horizon, y axis) which satisfied the willingness to pay threshold (x axis).
DISCUSSION
We found that in the US, PFO closure compared to medical therapy alone is a cost-effective secondary prevention strategy in young to middle aged patients with cryptogenic stroke. PFO closure surpassed our preset WTP threshold of $150,000, leading to better cost and QALY outcomes and an INMB of $52,761 in the base case.
The only parameter that would make PFO closure no longer cost-effective would be a decline in treatment effectiveness from a hazard ratio in the base case of 0.32 (or number needed to treat (NNT) 95/year) to a hazard ratio at or above 0.64 (NNT 345/ year). Of the five trials, only CLOSURE I exceeded the hazard ratio of 0.64, likely due to their inclusion of patients with TIA and lacunar strokes.4 In fact, the majority of recurrent strokes were due to etiologies other than paradoxical embolus. In addition, there are concerns regarding the efficacy of the STARFlex Septal Closure System which is now off the market in the US. In contrast, PC, RESPECT, CLOSE, and REDUCE only included patients with radiographic strokes, though 2.5% of patients in the PC trial had index events of systemic embolus.5 In the most efficacious trial, CLOSE (hazard ratio 0.03, NNT 7/year), only patients with atrial septal aneurysm or large shunt were enrolled. This magnitude of benefit was also seen in a subgroup of RESPECT with similar PFO characteristics. We chose to include all trials in this analysis to avoid bias, given the small number of published trials. These trial differences emphasize the need to more concisely define which patient populations will benefit most from PFO closure.
To our knowledge, there is one previously published cost-effectiveness model evaluating PFO closure.25 They used pooled results from the first 3 trials (CLOSURE I, PC, and RESPECT) and found a cost of $50,692 per QALY at 2.6 years. There are a few notable differences. First, TIA was used as an outcome and. Since there is no clinical test for TIA, it is generally an unreliable outcome to assess. Next, the utility of 0.52 assessed for every stroke may be overly pessimistic in this young population, which tends to have better recover.26 Lastly, the HR of stroke with closure was 0.7, resulting in a quicker onset of cost-effectiveness but longer time to reach dominance with PFO closure (30.2 years).
Procedure-related atrial fibrillation could affect the longer term cost-effectiveness of PFO closure, as atrial fibrillation could be an independent cause of strokes. Overall, atrial fibrillation was significantly more common in the closure arm than the medical arm. In CLOSURE I, 23 patients developed atrial fibrillation in the closure arm, 17 of which were transient and 6 were persistent.4 Similarly in CLOSE, only 3 of 11 patients who developed atrial fibrillation in the closure arm required continued anticoagulation8, and in RESPECT, peri-procedural atrial fibrillation was transient without recurrence. The risk of atrial fibrillation in the control arm was not zero in any trial, so there must be a proportion of patients in the closure arm that would have developed atrial fibrillation regardless of the procedure. Certainly, there is a significant risk of closure-induced atrial fibrillation, but whether this persists years after closure or if it is transient remains unclear. Hence, an initial cost and penalty of QALY for atrial fibrillation was taken at the beginning of this model without speculation into any potential later implications.
This model evaluates PFO closure from the healthcare payer perspective and additional costs such as loss of productivity were not accounted for. While the average age of first ischemic stroke in the US is 69–72 years, the average age of patients entering this model is 45.27 Unlike previous cost effectiveness models on stroke, these individuals are more likely to be in the prime of their careers, and stroke likely results in significant loss of potential income.28 While the mRS is a useful for grading stroke disability, it is biased toward gross motor disabilities. For example, a high functioning executive who has slowed processing speeds after stroke may have mRS 1 (slight disability, able to carry out usual tasks), but be unable to perform her previous job. Given these considerations, we chose the higher WTP threshold of $150,000/QALY, which has been recommended for the US.24
There are some limitations to this study. First, we assume that patients entered the model with a quality of life utility of 1 instead of discounting for the initial stroke. This is because, there is no data on utility after a cryptogenic stroke in younger patients nor on the utility after a recurrent stroke in this population. We introduced a wide sensitivity range to account for this uncertainty. To calculate the penalties from procedural complications we elected to use utilities complied in a large single source, which may not be the most up to date, instead of drawing from numerous disparate sources.13 Second, this economic evaluation is intended for young to middle aged cryptogenic stroke patients for whom the effects of atherosclerotic risk factors are not yet a major consideration. We do not factor in strokes from other causes, whose greater incidence would likely overshadow strokes from PFO later in life and overwhelm any benefit from PFO closure. Third, we estimated PFO procedural costs using the atrial septal defect closure procedural code which it is usually billed under. Although patients in this population are not yet old enough for Medicare, we used standardized Medicare costs because individual insurance plans can vary widely. Finally, cost of long term care was attained for an older stroke population and may not accurately reflect costs in a younger population.
In conclusion, PFO closure for cryptogenic strokes in the right setting is cost effective, producing benefit in QALYs and cost savings. However, patient selection remains vitally important as marginal declines in treatment effectiveness can dramatically affect cost effectiveness. In this setting, a multidisciplinary evaluation by a vascular neurologist in addition to the cardiologist may be helpful. Further work is needed to evaluate PFO closure in older adults and to more definitively identify, through patient-level meta-analysis, sub-groups that which may derive more benefit from treatment such as larger PFOs, presence of an atrial septal aneurysm or a venous hypercoagulability.
Supplementary Material
Acknowledgments
None.
Sources of Funding:
Michelle H. Leppert and Sharon N. Poisson are supported by the American Heart Association Bugher Foundation Grant. James F. Burke is supported by the NIH.
Footnotes
Disclosures:
John D. Carroll, MD and David E. Thaler, MD, PhD Consultant to Abbott while being steering committee member for RESPECT trial
References
- 1.Sacco RL, Ellenberg JH, Mohr JP, Tatemichi TK, Hier DB, Price TR, et al. Infarcts of undetermined cause: The nincds stroke data bank. Ann Neurol. 1989;25:382–390. doi: 10.1002/ana.410250410. [DOI] [PubMed] [Google Scholar]
- 2.Hagen PT, Scholz DG, Edwards WD. Incidence and size of patent foramen ovale during the first 10 decades of life: An autopsy study of 965 normal hearts. Mayo Clin Proc. 1984;59:17–20. doi: 10.1016/s0025-6196(12)60336-x. [DOI] [PubMed] [Google Scholar]
- 3.Lechat P, Mas JL, Lascault G, Loron P, Theard M, Klimczac M, et al. Prevalence of patent foramen ovale in patients with stroke. N Engl J Med. 1988;318:1148–1152. doi: 10.1056/NEJM198805053181802. [DOI] [PubMed] [Google Scholar]
- 4.Furlan AJ, Reisman M, Massaro J, Mauri L, Adams H, Albers GW, et al. Closure or medical therapy for cryptogenic stroke with patent foramen ovale. N Engl J Med. 2012;366:991–999. doi: 10.1056/NEJMoa1009639. [DOI] [PubMed] [Google Scholar]
- 5.Meier B, Kalesan B, Mattle HP, Khattab AA, Hildick-Smith D, Dudek D, et al. Percutaneous closure of patent foramen ovale in cryptogenic embolism. N Engl J Med. 2013;368:1083–1091. doi: 10.1056/NEJMoa1211716. [DOI] [PubMed] [Google Scholar]
- 6.Carroll JD, Saver JL, Thaler DE, Smalling RW, Berry S, MacDonald LA, et al. Closure of patent foramen ovale versus medical therapy after cryptogenic stroke. N Engl J Med. 2013;368:1092–1100. doi: 10.1056/NEJMoa1301440. [DOI] [PubMed] [Google Scholar]
- 7.Kent DM, Dahabreh IJ, Ruthazer R, Furlan AJ, Reisman M, Carroll JD, et al. Device closure of patent foramen ovale after stroke: Pooled analysis of completed randomized trials. J Am Coll Cardiol. 2016;67:907–917. doi: 10.1016/j.jacc.2015.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mas JL, Derumeaux G, Guillon B, Massardier E, Hosseini H, Mechtouff L, et al. Patent foramen ovale closure or anticoagulation vs. Antiplatelets after stroke. N Engl J Med. 2017;377:1011–1021. doi: 10.1056/NEJMoa1705915. [DOI] [PubMed] [Google Scholar]
- 9.Sondergaard L, Kasner SE, Rhodes JF, Andersen G, Iversen HK, Nielsen-Kudsk JE, et al. Patent foramen ovale closure or antiplatelet therapy for cryptogenic stroke. N Engl J Med. 2017;377:1033–1042. doi: 10.1056/NEJMoa1707404. [DOI] [PubMed] [Google Scholar]
- 10.Saver JL, Carroll JD, Thaler DE, Smalling RW, MacDonald LA, Marks DS, et al. Long-term outcomes of patent foramen ovale closure or medical therapy after stroke. N Engl J Med. 2017;377:1022–1032. doi: 10.1056/NEJMoa1610057. [DOI] [PubMed] [Google Scholar]
- 11.Leppert MH, Campbell JD, Simpson JR, Burke JF. Cost-effectiveness of intra-arterial treatment as an adjunct to intravenous tissue-type plasminogen activator for acute ischemic stroke. Stroke. 2015;46:1870–1876. doi: 10.1161/STROKEAHA.115.009779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Amplatzer™ pfo occluder for the prevention of recurrent ischemic stroke. Circulatory System Device Panel. 2016 [Google Scholar]
- 13.Tengs TO, Wallace A. One thousand health-related quality-of-life estimates. Med Care. 2000;38:583–637. doi: 10.1097/00005650-200006000-00004. [DOI] [PubMed] [Google Scholar]
- 14.Ganesalingam J, Pizzo E, Morris S, Sunderland T, Ames D, Lobotesis K. Cost-Utility Analysis of Mechanical Thrombectomy Using Stent Retrievers in Acute Ischemic Stroke. Stroke. 2015;46:2591–2598. doi: 10.1161/STROKEAHA.115.009396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Slot J, Berg E, Dorman P, Dennis M, Sandercock P. Impact of functional status at six months on long term survival in patients with ischaemic stroke: prospective cohort studies. BMJ. 2008;336:376–379. doi: 10.1136/bmj.39456.688333.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eriksson M, Norrving B, Terent A, Stegmayr B. Functional Outcome 3 Months after Stroke Predicts Long-Term Survival. Cerebrovasc Dis. 2008;25:423–429. doi: 10.1159/000121343. [DOI] [PubMed] [Google Scholar]
- 17.Brinjikji W, Rabinstein AA, Cloft HJ. Hospitalization Cost for Acute Ischemis Stroke Patients Treated With Intravenous Thrombolysis in the United States Are Substantially Higher Than Medicare Payments. Stroke. 2012;43:1131–1133. doi: 10.1161/STROKEAHA.111.636142. [DOI] [PubMed] [Google Scholar]
- 18.Shah A, Shewale A, Hayes CJ, Martin BC. Cost-effectiveness of oral anticoagulants for ischemic stroke prophylaxis among nonvalvular atrial fibrillation patients. Stroke. 2016;47:1555–1561. doi: 10.1161/STROKEAHA.115.012325. [DOI] [PubMed] [Google Scholar]
- 19.Shireman TI, Wang K, Saver JL, Goyal M, Bonafe A, Diener HC, et al. Cost-effectiveness of solitaire stent retriever thrombectomy for acute ischemic stroke: Results from the swift-prime trial (solitaire with the intention for thrombectomy as primary endovascular treatment for acute ischemic stroke) Stroke. 2017;48:379–387. doi: 10.1161/STROKEAHA.116.014735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.CMS. Center for medicare and medicaid services. Physician Fee Schedule Look-Up Tool. 2017 [Google Scholar]
- 21.HCUPNet. Healthcare cost and utilization project. Agency for Healthcare Research and Quality. [PubMed] [Google Scholar]
- 22.Sanders GD, Neumann PJ, Basu A, Brock DW, Feeny D, Krahn M, et al. Recommendations for conduct, methodological practices, and reporting of cost-effectiveness analyses: Second panel on cost-effectiveness in health and medicine. JAMA. 2016;316:1093–1103. doi: 10.1001/jama.2016.12195. [DOI] [PubMed] [Google Scholar]
- 23.Alastair Gray PC, Jane Wolstenholme, Sarah Wordsworth. Applied methods of cost-effectiveness analysis in health care. New York: Oxford Univerisyt Press; 2011. [Google Scholar]
- 24.Neumann PJ, Cohen JT, Weinstein MC. Updating cost-effectiveness — the curious resilience of the $50,000-per-qaly threshold. New England Journal of Medicine. 2014;371:796–797. doi: 10.1056/NEJMp1405158. [DOI] [PubMed] [Google Scholar]
- 25.Pickett CA, Villines TC, Ferguson MA, Hulten EA. Cost effectiveness of percutaneous closure versus medical therapy for cryptogenic stroke in patients with a patent foramen ovale. Am J Cardiol. 2014;114:1584–1589. doi: 10.1016/j.amjcard.2014.08.027. [DOI] [PubMed] [Google Scholar]
- 26.Nakayama H, Jorgensen HS, Raaschou HO, Olsen TS. The influence of age on stroke outcome. The copenhagen stroke study. Stroke. 1994;25:808–813. doi: 10.1161/01.str.25.4.808. [DOI] [PubMed] [Google Scholar]
- 27.Kissela BM, Khoury JC, Alwell K, Moomaw CJ, Woo D, Adeoye O, et al. Age at stroke: Temporal trends in stroke incidence in a large, biracial population. Neurology. 2012;79:1781–1787. doi: 10.1212/WNL.0b013e318270401d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leung LY, Melkumova E, Thaler DE. Longitudinal care for young adults with stroke. JAMA Neurol. 2017;74:1163–1164. doi: 10.1001/jamaneurol.2017.1874. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.