Abstract
Developmental stress, including low socioeconomic status (SES), can induce dysregulation of the hypothalamic-pituitary-adrenal axis and result in long-term changes in stress reactivity. Children in lower SES households experience more stress and are more likely to be exposed to environmental neurotoxins such as lead (Pb) and manganese (Mn) than children in higher SES households. Co-exposure to stress, Pb, and Mn during early development may increase the risk of central nervous system dysfunction compared with unexposed children. To investigate the potential interaction of these factors, Sprague-Dawley rats were bred, and litters born in-house were culled on postnatal day (P)1 to 6 males and 6 females. One male and female within each litter were assigned to one of the following groups: 0 (vehicle), 10 mg/kg Pb, 100 mg/kg Mn, or 10 mg/kg Pb + 100 mg/kg Mn (Pb-Mn), water gavage, and handled only from P4-28 with half the litters reared in cages with standard bedding (29 litters) and half with no bedding (Barren; 27 litters). Mn and Pb-Mn groups had decreased anxiety, reduced acoustic startle, initial open-field hypoactivity, increased activity following (+)-methamphetamine, deficits in egocentric learning in the Cincinnati water maze (CWM), and deficits in latent inhibition conditioning. Pb increased anxiety and reduced open-field activity. Barren-reared rats had decreased anxiety, CWM deficits, increased startle, and initial open-field hyperactivity. Mn, Pb-Mn, Pb Barren-reared groups had impaired Morris water maze performance. Pb altered neostriatal serotonin and norepinephrine, Mn increased hippocampal serotonin in males, Mn + Barren-rearing increased neostriatal serotonin, and Barren-rearing decreased neostriatal dopamine in males. At the doses used here, most effects were in the Mn and Pb-Mn groups. Few interactions between Mn, Pb, and rearing stress were found, indicating that the interaction of these three variables is not as impactful as hypothesized.
Introduction
Chronic stress, particularly during development, can impair neural functioning through dysregulation of the hypothalamic-pituitary-adrenal (HPA) axis leading to neuronal damage (Lupien et al., 1999; McEwen et al., 1992; McEwen and Stellar, 1993; McEwen, 1998). During development the HPA axis undergoes a period of blunted response to stress: the stress hyporesponsive period (SHRP). The SHRP is hypothesized to be neuroprotective against excitotoxicity during critical periods of brain development (De Kloet et al., 1988; Sapolsky and Meaney, 1986). However, the SHRP has limited capacity; stressors that exceed its buffering capacity pose risks for long-term effects (Anisman et al., 1998; Gos et al., 2008; Gruss et al., 2008). One determinate of childhood stress is low socioeconomic status (SES). Children raised in low SES environments often experience family and neighborhood problems, such as family disruption, hunger, violence, and neglect; such children show elevated cortisol levels (Cohen et al., 2006; Gump et al., 2008; Lupien et al., 2000; Lupien et al., 2001).
In addition to chronic stress, children in such environments may also be exposed to heavy and transition metals. Lead (Pb) is found in older housing in paint, dust, and soil (Jacobs et al., 2002; Levin et al., 2008; Muntner et al., 2005; Schnaas et al., 2004). High blood Pb levels (BPb) in children are associated with encephalopathy (Patel and Athawale, 2009), altered cardiovascular function (Gump et al., 2005), and genotoxicity (Mendez-Gomez et al., 2008); at moderate BPb levels, other symptoms appear: attention deficit disorders (Chiodo et al., 2007; Nigg et al., 2008; Surkan et al., 2007), decreased gray matter (Cecil et al., 2008), lower IQ (Chiodo et al., 2007; Needleman et al., 1979; Surkan et al., 2007; Wang et al., 1989), impaired language (Yuan et al., 2006), social and behavioral problems (Burns et al., 1999; Chiodo et al., 2007), increased delinquent behavior (Dietrich et al., 2001), and higher rates of arrest (Wright et al., 2008). Many of the aforementioned deficits are found in children with BPb levels below 5 μg/dL (Centers for Disease Control and Prevention, 2005; Centers for Disease Control and Prevention, 2012); a safe level of Pb has yet to be defined (Bellinger, 2011; Canfield et al., 2003; Lanphear et al., 2005). Pb can also affect the stress response. Prenatal stress and Pb together increase the negative impact of Pb in rats on brain and behavior (Cory-Slechta et al., 2010; Cory-Slechta et al., 2012; Cory-Slechta et al., 2013a; Cory-Slechta et al., 2013b; Virgolini et al., 2008). Rats exposed to Pb postnatally, as a model of early childhood exposure, in combination with stress had decreased thymus and spleen weights and altered brain monoamines (Amos-Kroohs et al., 2016b; Graham et al., 2011).
Lower SES children are also at increased risk of exposure to manganese (Mn). Soy-based baby formulas can contain up to 10 times the amount of Mn in dairy-based formulas and 100 times more than in human milk (Aschner and Aschner, 2005; Collipp et al., 1983; Lonnerdal, 1994; Tran et al., 2002a; Tran et al., 2002b). Children from lower SES backgrounds are more likely to be fed soy-based formulas and are more likely to be exposed to Mn in air, soil, and water from industrial or ground water sources (Bouchard et al., 2011; Henn et al., 2014; Hu et al., 2007; Khan et al., 2012; Lucchini et al., 2012; Naess et al., 2007). Although an essential nutrient in low doses, excess Mn (Mn over-exposure (MnOE)) has adverse effects (Aschner and Aschner, 2005; Racette et al., 2012; Roth, 2009). In rodents developmental MnOE results in cognitive deficits, altered locomotor activity, and blunted acoustic startle responses (Amos-Kroohs et al., 2015; Amos-Kroohs et al., 2016a; Amos-Kroohs et al., 2017; Tran et al., 2002b). MnOE in children is associated with decreased IQ, reduced school performance, and behavioral disinhibition (Bouchard et al., 2011; Khan et al., 2011; Khan et al., 2012; Lucchini et al., 2012). MnOE and stress may interact to alter brain development in rodents as seen by increased mortality, altered corticosterone levels, reduced body weight gain, and monoamine changes (Vorhees et al., 2014).
Children with simultaneous exposure to Pb and stress, Mn and stress, or all three may be at particularly high risk for neural and behavioral impairments. For example, BPb levels are positively correlated with blood Mn in children in urban environments or near industrial facilities (Delves et al., 1973; Joselow et al., 1978; Zielhuis et al., 1978), and the combination of Pb, Mn, and stress is documented (Carlin et al., 2013; Hu et al., 2007; Sanders et al., 2015; Sexton et al., 2006). The purpose of this study was to test the hypothesis that simultaneous exposure to stress, Pb, and Mn during development interact to adversely affect cognition, behavior, and monoamines. We used barren cage rearing, a model of developmental stress that causes long-term effects and models environmental impoverishment (Baum et al., 1999; Gilles et al., 1996; Ivy et al., 2008; Rice et al., 2008). Barren cage rearing in combination with developmental Pb (Graham et al., 2011) or MnOE (Vorhees et al., 2014) affected organ weight, corticosterone, monoamines, and growth (Amos-Kroohs et al., 2016a; Amos-Kroohs et al., 2016b). Herein, rats were treated with Pb (10 mg/kg), Mn (100 mg/kg), or both (Pb-Mn) in barren or standard cages during a period of brain development analogous to late gestation to early childhood (Clancy et al., 2001; Clancy et al., 2007a; Clancy et al., 2007b). The doses of Pb and Mn used produce blood levels in the range of BPb and BMn found in children (Amos-Kroohs et al., 2016b; Bellinger, 2011; Graham et al., 2011; Lanphear et al., 2005; Vorhees et al., 2014). The hypothesis was that developmental exposure to Pb, Mn, and stress together would interact and lead to more severe effects on behavior and monoamines than any of the independent variables given alone or in combinations of two.
Materials and Methods
Animals
All procedures were approved by the Cincinnati Children’s Research Foundation Institutional Animal Care and Use Committee and conformed to guidelines of the National Institutes of Health for the care and use of animals in research. Rats were maintained in an AAALAC International accredited vivarium on a 14:10 h light-dark cycle (lights on at 600 h) with controlled temperature (19 ± 1 °C) and humidity (50 ± 10%). NIH-07 rodent chow with consistent levels of metals and minerals was provided ad lib along with reverse osmosis filtered, UV sterilized water. Male and nulliparous female Sprague-Dawley CD (IGS) rats (strain 001, Charles River Laboratories, Raleigh, NC) were acclimated to the vivarium for at least one week prior to cohabitation. The morning a sperm plug was detected was designated embryonic day 0 (E0). On E1 females were transferred to polycarbonate cages (26 × 48 cm and 20 cm tall) containing woodchip bedding and a semicircular stainless steel enclosure as environmental enrichment (Vorhees et al., 2008). Day of birth was designated postnatal day (P0). On P1 litters were culled to 12 pups (6 males and 6 females) using a random number table. If a litter had only 10 or 11 pups, one or two pups from another litter born the same day was in-fostered to make the litter complete. Rats were identified by ear punch on P4. Sex and body weights were recorded on P1 and every other day from P4-28 and weekly thereafter.
Stressor Administration
Fifty-six litters were used: 29 litters with standard cage bedding and 27 litters in barren cages (Gilles et al., 1996; Graham et al., 2011; Vorhees et al., 2014). For barren cages, woodchip bedding was removed and replaced with a single paper towel (changed daily). Rats were divided and transferred to new barren or standard cages from P4-28. Thereafter, all rats were housed in standard cages.
Metal Administration
Within each litter, one male and one female were randomly assigned to receive one of the following treatments by gavage: vehicle (VEH), lead (Pb), manganese (Mn), Pb and Mn (Pb-Mn), distilled water (H2O), or handling only (HAND). The dosing volume of 3 mL/kg was gavaged using a 24-gauge flexible tube with a ball tip from P4-28 every other day. The Pb dose was 10 mg/kg Pb acetate (expressed as the free metal) in 0.01 M anhydrous sodium acetate. The Mn dose was 100 mg/kg Mn chloride tetrahydrate dissolved in distilled H2O (Sigma, St. Louis, MO). The Pb-Mn dose was 10 mg/kg Pb acetate + 100 mg/kg Mn dissolved in vehicle. VEH was sodium acetate/chloride (acetate=0.9 mg/mL, chloride=21 mg/mL in H2O). The vehicle solution was made by first dissolving 1.25 mg sodium acetate in water until fully dissolved and then 34 mg sodium chloride was added until it was fully dissolved. The water control was distilled H2O. Littermates assigned to the HAND group were removed from the cage, weighed, and handled for the same amount of time as treated pups. We have shown that gavage by experienced personnel does not significantly increase plasma corticosterone levels in pups compared with non-gavaged pups at these ages (Graham et al., 2011). Gavage was used to provide control over dose compared with methods that put metals in drinking water. On treatment days, rats were gavaged between 1000 and 1400 h. Dams were removed from litters on P28, and pups were placed in standard cages in same-sex pairs. Rats were tested by personnel blind to treatment group assignment. Given that rats engage in corprophagia, there exists the potential for some minor cross-group exposure to Mn or Pb in a split-litter design. However, Graham et al. (2011) used a split-litter design and showed that on P29 control rats had little to no Pb in whole blood compared with rats that were dosed with 1 or 10 mg/kg Pb in a regimen consistent with the current study.
Behavior
Testing began on P60 and testing was done in the following order: elevated zero maze (EZM), light/dark test, open-field, prepulse inhibition (PPI) of acoustic startle, straight swim channel, Cincinnati water maze (CWM), Morris water maze (MWM), latent inhibition, and open-field with methamphetamine challenge. Equipment was cleaned between subjects with a 70% EtOH solution.
Elevated Zero Maze
Anxiety-like behavior was measured using an EZM (Braun et al., 2011). The apparatus consisted of a black circular acrylic track 10 cm wide, 105 cm i.d., and elevated 72 cm above the floor (San Diego Instruments, San Diego, CA). Testing took place under 12 lux illumination. A camera was mounted above and connected to a monitor in an adjoining room. Rats were placed in the middle of a closed quadrant and scored for 5 min by an observer who scored the behavior. Dependent variables were latency to first open quadrant entry, time in open quadrants, number of open quadrant entries, and number of head dips.
Light↔Dark Test
On P61 rats were tested in 40 × 40 × 16 cm high acrylic test arenas (AccuScan Instruments Inc., Columbus, OH) with a dark box inserted that occupied one-half of the space measuring 20 × 40 × 16 cm high with an opening connecting the two sides. Rats started in the light side. The number of transitions, latency to first dark side entry, and the total time spent on each side were recorded for 10 min.
Open-field
On P62 rats were placed in 40 cm × 40 cm × 16 cm high open-field arenas for 60 min (Accuscan Instruments, Columbus, OH). The total number of horizontal infrared photobeam interruptions and time in the center of the arenas were recorded, and data analyzed in 5 min intervals.
PPI of Acoustic Startle
On P63 acoustic startle with PPI was assessed. Responses were measured in SR-LAB apparatus (San Diego Instruments, San Diego, CA). Each rat was placed in an acrylic cylindrical holder mounted on a platform with a piezoelectric accelerometer attached to the underside; the platforms were located inside sound-attenuated chambers with house light and fan. A 5 min acclimation period preceded test trials. Each rat received a 4 × 4 Latin square sequence of 4 trial types: No stimulus, pulse (110 dB), 70 dB prepulse + pulse, or 76 dB prepulse + pulse. Each set of 16 trials was repeated 3 times for a total of 48 trials. Trials of the same type were averaged together. Pulses were 20 ms, 110 dB SPL, mixed frequency signals with 1.5 ms rise time. The recording window was 100 ms. Prepulses preceded pulses by 70 ms (onset to onset), and the intertrial interval (ITI) was 8 s. Maximum startle amplitude (Vmax) and average amplitude (both in mV) were recorded, and data for Vmax are reported since both revealed the same effects.
Straight Channel
On P64 rats were tested in a 244 cm × 15 cm wide × 50 cm high channel filled halfway with water for four trials under standard room lighting. Latency to reach a submerged platform at the opposite end was recorded. Straight channel trials acclimate rats to swimming, teach that escape is on a submerged platform, and the data are used to ensure that swimming ability and motivation to escape are comparable across groups.
Cincinnati Water Maze
To assess egocentric learning (Braun et al., 2012; Braun et al., 2015; Braun et al., 2016), rats were tested in the CWM starting on P65. The CWM is a 9-unit multiple T-maze with a complex path from start to goal and T-shaped cul-de-sacs that branch from the main channel. The goal arm has a submerged escape platform (Vorhees, 1987; Vorhees et al., 2008; Vorhees and Williams, 2016). Water was maintained at 21 ± 1 °C. To prevent use of distal cues, testing was conducted under infrared light. An infrared sensitive camera was mounted above the maze and connected to a monitor in an adjoining room. Rats were acclimated to the dark for at least 5 min before testing. At the beginning of each trial, a rat was placed in the maze and allowed to search for the goal for up to 5 min. Errors (defined as a head and shoulder entry into a stem or arm of a T) and latency were recorded. Rats were tested for 18 days, 2 trials/day. Rats not reaching the goal within 5 min on trial-1 of a given day were removed, placed in a cage with absorbent material to wick away water, and allowed to rest for at least 5 min before being given trial-2. If the goal was found in < 5 min on trial-1, the rat was given trial-2 immediately.
Morris Water Maze
To assess allocentric hippocampal-mediated learning and memory, rats were tested in the MWM (Vorhees and Williams, 2006). The circular tank was 210 cm diameter × 51 cm deep made of stainless steel, painted black inside, and filled with water to a depth of 25 cm. There were curtains mounted around the tank that could be closed to hide distal cues; when the curtains were open, large geometric shapes mounted on the walls were visible. Rats were tested in phases: (1) acquisition (P83-89), (2) reversal (P90-96), (3) shift (P97-103), and (4) cued-random (P104-105). For the first three phases, curtains were open. The procedure consisted of 4 trials per day for 6 days to find a hidden, submerged platform, followed 24 h later by a probe trial with no platform. Probe trials were 30 s; hidden platform trials had a 2 min limit with an ITI of 15 s on the platform. Rats that did not find the platform within the limit were placed on it. The platform was submerged ~2 cm below the water surface and positioned equidistant between the center and wall of the tank. For acquisition a 10 cm diameter platform was placed in the SW quadrant. Rats were started at one of four pseudorandom positions around the perimeter (Vorhees and Williams, 2006). During reversal a 7 cm diameter platform was placed in the NE quadrant. During shift a 5 cm diameter platform was placed in the NW quadrant. A camera mounted above the maze was synchronized to a computer with video tracking software (SMART Track, Harvard Apparatus, Holliston, MA). For learning trials, dependent variables were latency, swim speed, path length, and cumulative distance from the platform. Dependent measures on probe trials were time in the target quadrant, average distance to the former platform site, and swim speed.
Morris Water Maze Cued
Cued-random trials were conducted for two days after shift (P104-105). Curtains were closed around the tank to block distal cues. A yellow plastic ball was affixed to a metal rod that protruded 10 cm above the water and was mounted in the center of the platform. Rats were given 4 trials/day for 2 days. The positions of the platform and start location were pseudorandomized on every trial to prevent use of spatial cues.
Morris Water Maze Cue Rotation
Cue rotation started on P106. This was done to test whether rats were following the experimental distal cues or inadvertent cues within the space that are difficult to control, such as those on the ceiling, variations in lighting, etc. The 5 cm diameter platform was moved to the SE quadrant, and a different set of start locations were used. Curtains were closed and three geometric cues were mounted at the edge of the tank slightly above the edge where they were visible from water level. Cues were placed in quadrants where the platform was not located. Rats were tested for 6 days (4 trials/day). On day-7 the cues were rotated 180° while the platform remained in the same location.
Morris Water Maze with Distractor Cues
This phase started on P113. The first 6 days were identical to those used for the distinctive cue phase except that on day-7, 4 trials were given with 4 irrelevant cues added as distractors.
Latent Inhibition
On P114, rats were placed in conditioned freezing test chambers (Coulbourn Instruments, Allentown, PA). Rats in each group were subdivided in two conditions each lasting 15 min: (a) tone pre-exposed (PE) and (b) not tone pre-exposed (NPE). PE rats were placed in a chamber and received 30 tones (82 dB, 2 kHz, 30 s duration) separated by 30 s intervals for 15 min. NPE rats received no tone during a comparable 15 min session. Following this rats were given 3 tone-footshock pairings (180 s between CS-US pairings) of 0.5 mA lasting 1 s. Contextual conditioning was assessed 24 h later. Rats were returned to the chamber for 6 min and freezing measured. Twenty-four hours later, rats were placed in the chambers with a different floor (grid rather than bars). Rats were assessed for 3 min with no-tone followed by 3 min with continuous tone. FreezeFrame software (Coulbourn Instruments) was used to determine the percentage of time spent freezing.
Open-field Activity with Methamphetamine
On P117, rats were placed in 40 × 40 cm activity monitors (AccuScan Instruments, Columbus, OH) for 30 min to re-habituate them to the apparatus. Following this they were removed and administered (+)-methamphetamine HCl (1 mg/kg, freebase in 3 mL/kg, Sigma-Aldrich) s.c. and tested for an additional 120 min.
Monoamines
A separate cohort of litters (n = 8/cage condition) were bred and treated as above. At P60 these rats were decapitated and neostriatum and hippocampus dissected over ice, rapidly frozen, and stored at −80 °C until analyzed for monoamines by high performance liquid chromatography with electrochemical detection (HPLC-ECD). Tissue was weighed and sonicated in ice-cold 0.1 N perchloric acid and centrifuged at 20,800 RCF for 13 min at 4 °C. The supernatant was collected and loaded (20 μL) onto a Dionex UltiMate 3000 Analytical Autosampler (Thermo Scientific, Waltham, MA). The mobile phase (pH = 3) was MD-TM Mobile Phase (Thermo Fisher Scientific) and consisted of 89% water, 10% acetonitrile, and 1% sodium phosphate monobasic (monohydrate). An ESA 5840 pump with a flow rate of 0.9 mL/min and temperature maintained at 28 °C was used. The system included a guard cell set to +350 mV, a Supelco Supelcosil LC-18 column (15 cm × 4.6 mm, 3 μm; Sigma-Aldrich) and a Coulochem III electrochemical detector (Thermo Scientific) with potential settings set to −150 mV for E1 and +250 mV for E2. Neurotransmitter concentrations for dopamine (DA), 3,4-dihydroxyphenylacetic acid (DOPAC), homovanillic acid (HVA), serotonin (5-HT), 5-hydroxyindolacetic acid (5-HIAA), and norepinephrine (NE) were calculated from serial dilutions.
Statistical Analyses
Data were analyzed using mixed linear ANOVAs (SAS 9.3, Proc Mixed, SAS Institute, Cary, NC). Fixed factors were exposure group (Control, Pb, Mn, and Pb-Mn), cage rearing (Standard or Barren), and sex. Preliminary analyses found no differences among the three control groups (VEH, H2O, and HAND); thus, these groups were combined into a single control group. To account for litter effects, litter was a randomized block factor in the ANOVA models. For models with a repeated measure, the autoregressive-1 covariance structure was used. Linear mixed ANOVAs fit data to a maximum likelihood model by comparing outcomes with each factor added stepwise. In these models, the Kenward-Rogers first-order adjusted degrees is used to obtain F-ratios. The autoregressive-1 model was chosen after using the Akaike information criterion with correction (AICC) statistic to determine the model of best fit to the data. Slice-effect ANOVAs were used to analyze interactions. Tukey-Kramer tests were used for post hoc pairwise comparisons where significant F-values were obtained. The hypothesis was that metal and barren cage exposure would induce cognitive deficits based on our prior data; therefore, these outcomes were tested directionally (1-tailed); all others were tested 2-tailed. Significance was set at p ≤ 0.05. Data are presented as least square (LS) mean ± SEM.
Results
Preweaning Weights
A main effect of cage (F(1,62) = 26.95, p < 0.001) revealed that Barren-reared rats weighed less than Standard-reared rats (Barren: 32.7 ± 0.8 g, Standard: 38.6 ± 0.8 g). An effect of exposure (F(3,546) = 65.64, p < 0.001) and an exposure × day interaction (F(36,7208) = 22.50, p < 0.001) were found, as well as an exposure × cage × day interaction (F(36,7208) = 1.94, p < 0.001). Analysis of the 3-way interaction revealed Standard-reared Pb-Mn rats weighed less than Controls from P8-28 and Mn rats weighed less from P10-28 (Figure 1A). In Barren-reared rats, Pb-Mn and Mn rats weighed less than Controls from P12-28 and from P10-28, respectively (Figure 1B).
Fig. 1. Preweaning body weight.
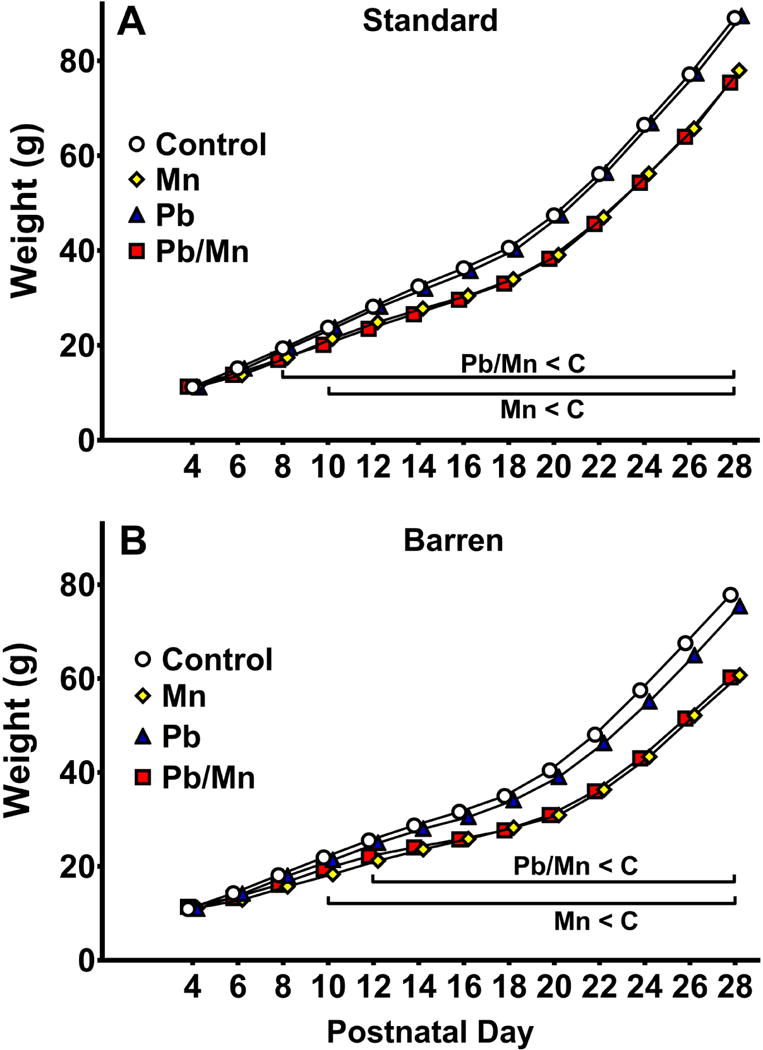
A: Preweaning body weight (g) in in the Standard-reared groups. Pb-Mn and Mn rats weighed less than Controls from P8-28 and P10-28, respectively (p < 0.05 to p < 0.001). B: Preweaning body weight (g) in the Barren-reared groups. Pb-Mn and Mn rats weighed less than Controls from P12-28 and P10-28, respectively (p < 0.05 to p < 0.001). Group sizes: Total (M/F): Standard Control = 175 (86/89); Standard Mn = 56 (29/27); Standard Pb-Mn = 50 (26/24); Standard Pb = 56 (28/28); Barren Control = 158 (81/77); Barren Mn = 41 (19/22); Barren Pb-Mn = 37 (17/20); Barren Pb = 51 (27/24). Data are LS Mean ± SEM. Bar indicates ages where significant group differences were found.
Behavior
Elevated Zero Maze
For time spent in open quadrants, there was a main effect of exposure (F(3,357) = 9.43, p < 0.001) wherein the Pb-Mn and Mn rats spent more time in the open than Pb-exposed or Control groups (p < 0.05-0.001). Exposure × sex (F(3,357) = 2.67, p < 0.05), exposure × cage (F(3,357) = 2.69, p < 0.05), cage × sex (F(1,357) = 5.87, p < 0.01), and exposure × cage × sex interactions (F(3,357) = 3.03, p < 0.05) were found. Post hoc analysis of the 3-way interaction revealed that for standard-reared male rats, the order of effects for time in open was Pb < Control males < Pb-Mn = Mn males (p < 0.05–0.001; Figure 2A). For standard-reared females, the order was Pb-Mn > than Control for time spent in the open (p < 0.05; Figure 2A). For Barren-reared rats, the order for males was Mn > other groups (p < 0.01–0.001; Figure 2B). There were no differences in Barren-reared females (Figure 2B).
Fig. 2. Elevated zero maze (EZM).
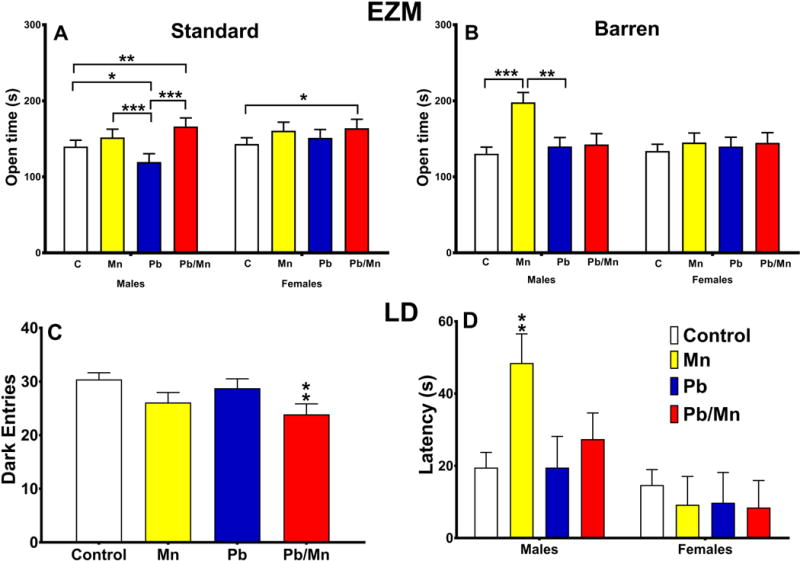
A: Exposure × sex interaction for time spent in the open quadrants for Standard-reared rats; B: Exposure × sex interaction for time spent in the open quadrants for the Barren-reared rats. Group sizes: Total (M/F): Standard Control = 115 (56/59); Standard Mn = 38 (20/18); Standard Pb-Mn = 34 (18/16); Standard Pb = 38 (19/19); Barren Control = 101 (54/47); Barren Mn = 27 (13/14); Barren Pb-Mn = 22 (10/12); Barren Pb = 32 (17/15). Data are LS Mean ± SEM. Light Dark (LD) test: C: Main effect of exposure for number of dark entries; D: Exposure × sex interaction for latency (s) to enter the dark chamber. Group sizes: Total (M/F): Control = 218 (110/108); Mn = 64 (32/32); Pb-Mn = 55 (27/28); Pb = 72 (37/35). Data are LS Mean ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001 vs. Controls.
For head dips, a main effect of exposure (F(3,357) = 5.77, p < 0.001) revealed that Mn rats had more head dips than Controls (p < 0.001; Mn: 14.7 ± 1.4; Control: 11.6 ± 1.3). There was also an exposure × cage × sex interaction (F(3,357) = 3.59, p < 0.01); post hoc analysis revealed that Barren-reared Mn males made more head dips than Control Barren-reared males (p < 0.01; Barren Males: Mn: 18.7 ± 2.4; Control: 9.4 ± 1.9); no differences were found among Standard-reared groups or Barren-reared females. There were no effects on latency to first open quadrant entry or number of quadrant entries.
Light↔Dark Test
For the number of dark entries, a main effect of exposure (F(3,361) = 4.60, p < 0.01) revealed that Pb-Mn (p < 0.01) rats made fewer dark entries compared with Controls (Figure 2C). For latency there was an exposure × sex interaction (F(3,393) = 2.58, p < 0.05). Post hoc analysis revealed that Mn males took longer to enter the dark compared with Control males (p < 0.01; Figure 2D). There was also a main effect of cage on latency (F(1,393) = 16.77, p < 0.001) and a cage × sex interaction (F(1,393) = 10.70, p < 0.001). Slice-effect tests revealed that Barren-reared males had shorter latencies than Standard-reared males (p < 0.01; Barren: 10.0 ± 5.5 s, Standard: 47.5 ± 4.8 s); there were no differences for females. For time in the light, there was a cage × sex interaction (F(1,361) = 5.97, p < 0.01), although the post-hoc test showed no differences.
Open-field
There was no main effect of exposure on open-field activity, but there was an exposure × interval interaction (F(33,4279) = 1.91, p < 0.001). The Pb-Mn rats were less active than Controls from 0–10 min but more active from 40–45 min. The Mn and Pb rats were less active during the first 5 min compared with Controls regardless of cage (p < 0.05–0.01; Figure 3A,B). There was also a cage × interval interaction (F(11,3917) = 1.96, p < 0.05); the latter revealed that Barren-reared rats were more active than Standard-reared rats during the first 5 min (p < 0.01; Barren: 2198.4 ± 56.6 beam breaks; Standard: 2001.2 ± 50.8 beam breaks. There were no effects of exposure or cage on center time.
Fig. 3. Open-field (OF) exploration.
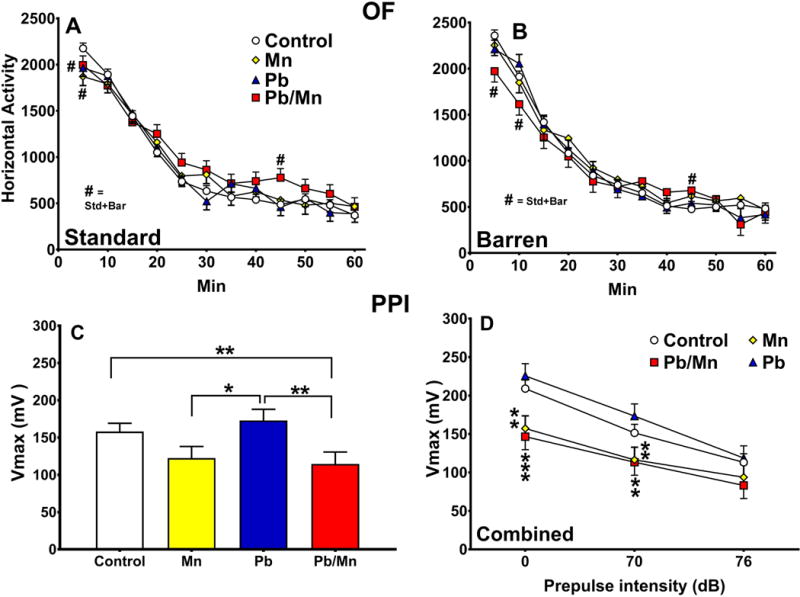
A: Activity in 5-min intervals in Standard-reared rats; B: Activity in 5-min intervals in Barren-reared rats. Exposure group sizes: Total (M/F): Standard Control = 122 (60/62); Standard Mn = 36 (19/17); Standard Pb-Mn = 35 (19/16); Standard Pb = 40 (20/20); Barren Control = 111 (58/53); Barren Mn = 27 (12/15); Barren Pb-Mn = 24 (11/13); Barren Pb = 35 (19/16). See text for details. Acoustic startle with prepulse inhibition (ASR/PPI): Peak response amplitude (Vmax) in mV. C: Main effect of exposure; D: Effect by prepulse with Standard- and Barren-reared groups combined. Exposure group sizes: Total (M/F): Control = 221 (112/109); Mn = 64 (32/32); Pb-Mn = 59 (29/30); Pb = 69 (36/33). Data are LS Mean ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001 vs. Controls.
PPI of Acoustic Startle
There was a main effect of exposure on maximum startle amplitude (F(3,372) = 5.52, p < 0.001). The Pb-Mn group had reduced Vmax compared with Pb and Control groups (p < 0.01) and the Mn group had reduced Vmax compared with Pb (p < 0.05; Figure 3C). There was also an exposure × PPI interaction (F(6,792) = 2.32, p < 0.05), that revealed that at PP-0, Mn and Pb-Mn rats had reduced Vmax compared with Pb and Control rats (p < 0.01–0.001); at PP-70, Pb-Mn and Mn rats had reduced Vmax compared with Pb rats (p < 0.01); at PP-76 there were no differences (Figure 3D).
Straight Channel
There was a main effect of exposure (F(3,1624) = 4.44, p < 0.01) in which the Mn group took longer to reach the platform compared with Controls (p < 0.01; Controls: 14.6 ± 0.5 s, Mn: 17.1 ± 0.7 s, Pb: 14.8 ± 0.7 s, Pb-Mn: 15.3 ± 0.7 s), however, there were no differences on the last trial, indicating that the groups had achieved equal swim speeds by the time they began the CWM.
Cincinnati Water Maze
For errors, a main effect of exposure (F(3,409) = 9.56, p < 0.001) revealed that Mn and Pb-Mn rats made more errors than Pb and Control rats (p < 0.05–0.001; Figure 4A,B). There was an exposure × sex interaction (F(3,410) = 5.30, p < 0.001) wherein the Mn and Pb-Mn females made more errors than Pb and Control females (p < 0.01–0.001; Control: 17.8 ± 0.9, Pb: 18.6 ± 1.6, Mn: 25.2 ± 1.6, Pb-Mn: 28.6 ± 1.7). No differences were found for males. An exposure × day interaction (F(51,6749) = 1.58, p < 0.01) revealed that Pb-Mn rats made more errors than Controls on days 4–9, and Mn rats made more errors than Controls on days 5–14 and 16 (Figure 4A). There was an effect of cage (F(1,62.2) = 17.58, p < 0.001: cf. Figure 4C & D) with Barren-reared rats making more errors than Standard-reared rats (Figure 4C inset) and a cage × day interaction (F(17,6335) = 4.05, p < 0.001; not shown) attributable to Barren-reared rats making more errors than Standard-reared rats on days 5–16 (p < 0.10–0.001).
Fig. 4. Cincinnati water maze (CWM) errors.
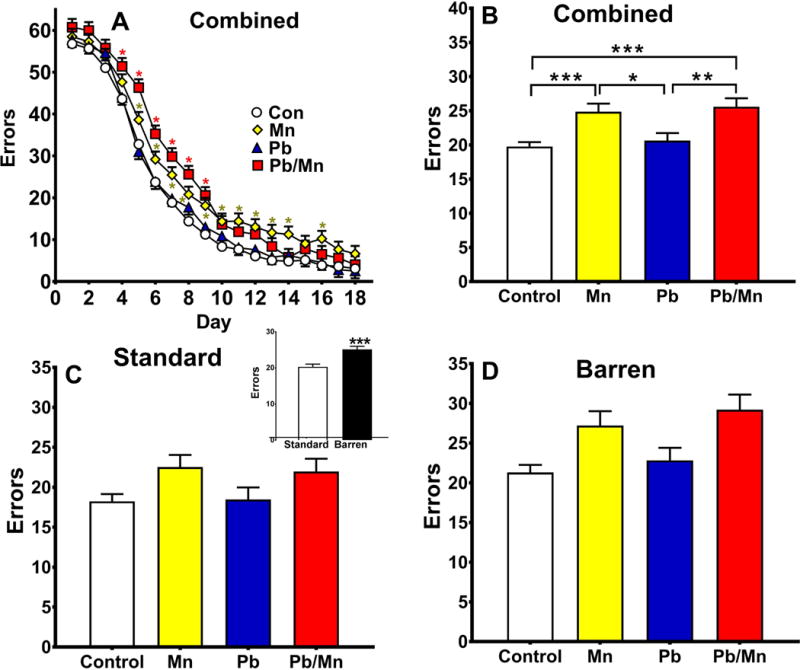
A: Errors by day with cage factor combined. Pb-Mn rats made more errors than Controls on days 4–9 (p < 0.05 to p < 0.001). Mn rats made more errors than Controls on days 5–14 and 16 (p < 0.01 to p < 0.001); B: Main effect of exposure; C: Errors in Standard-reared rats, inset is Standard vs Barren main effect; D: Errors in Barren-reared rats. Group sizes: Total (M/F): Standard Control = 121 (59/62); Standard Mn = 39 (21/18); Standard Pb-Mn = 35 (19/16); Standard Pb = 39 (19/20); Barren Control = 111 (58/53); Barren Mn = 27 (12/15); Barren Pb-Mn = 24 (11/13); Barren Pb = 35 (19/16). Data are LS Mean ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001.
For latency, the pattern was similar (not shown). There was a main effect of exposure (F(3,401) = 5.71, p < 0.001) that revealed that Mn and Pb-Mn rats took longer to find the platform compared with Controls or Pb (p < 0.05–0.01). An exposure × sex interaction (F(3,402) = 4.58, p < 0.01) revealed that the Mn and Pb-Mn females had longer latencies compared with Pb and Control females (p < 0.05–001; Control: 98.5 ± 4.2 s, Pb: 102.1 ± 7.4 s, Mn: 128.0 ± 7.6 s, Pb-Mn: 140.2 ± 8.3 s); no exposure-related differences were found in males. An exposure × day interaction (F(51,6356) = 1.47, p < 0.05) revealed that Pb-Mn rats on days 5–10 and Mn rats on days 7–14 and 16 had longer latencies than Controls. There was an effect of cage (F(1,61.9) = 25.50, p < 0.001) that revealed Barren-reared rats had longer latencies than Standard-reared rats and a cage × day interaction (F(17,5937) = 4.10, p < 0.001); the interaction revealed that Barren-reared rats had longer latencies than Standard-reared rats on days 5–16 (p < 0.05–0.001); exposure did not interact with cage.
Morris Water Maze Hidden Platform
Acquisition
For latency, path length, cumulative distance, and swim speed there were no main effects of exposure or cage, but there were exposure × cage × day interactions for each (F(15, 1841) = 1.59, p < 0.05), (F(15, 1843) = 1.61, p < 0.05), and (F(15,1833) = 1.72, p < 0.05), respectively. There were also exposure × sex × cage × day interactions on latency (F(15, 1841) = 1.63, p < 0.05), path length (F(15, 1843) = 1.58, p < 0.05 (Figure 5A–D)), and cumulative distance (F(15, 1833) = 1.69, p < 0.05). Post hoc analyses revealed that Standard-reared Mn males had longer latencies than Standard Control males on day-1, and Standard Pb-Mn males had longer latencies than Control males on day-2 (both p < 0.05; not shown). For path length (Figure 5A), Standard-reared Mn males had longer paths than Controls on day-1, Pb-Mn males had longer path lengths on days 1 and 2, and Pb males had longer paths on day-2 (p < 0.05). In Standard-reared females, Pb-Mn rats had longer paths than Mn, Pb, and Control females on day-1 (p < 0.05–0.01; Figure 5C). No differences were found for path length for Barren-reared males or females (Figure 5B,D). For cumulative distance, Standard-reared Mn males were farther from the platform than Standard Control males on day-1, and Standard Pb-Mn males were farther from the platform than Standard Control males on day-2 (p < 0.05–0.01); also, Standard-reared Pb-Mn females were farther from the platform than Standard Pb females on day-1 (p < 0.01; not shown). No differences were found in Barren-reared rats.
Fig. 5. MWM acquisition.
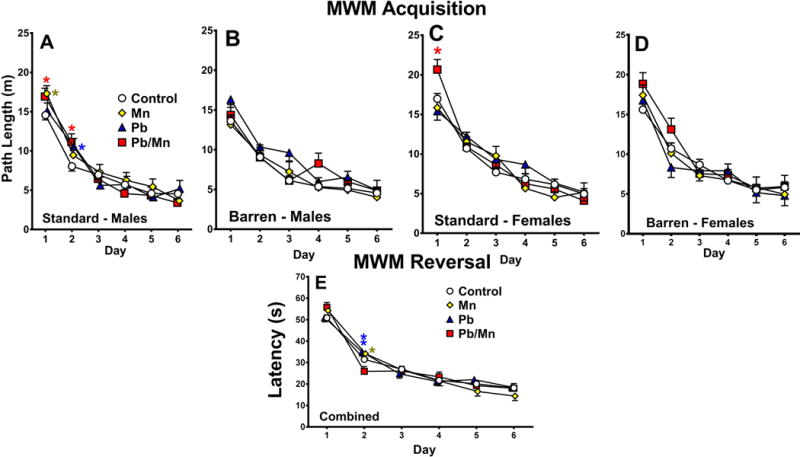
A: Path length (m) by day for Standard-reared males; *p < 0.05 vs. Controls; B: Path length (m) by day for Barren-reared males; C: Path length (m) by day for Standard-reared females; *p < 0.05 for Pb-Mn vs. Controls (or Mn and Pb); D: Path length (m) by day for Barren-reared females. MWM reversal: E: Latency (s) by day with cage condition combined; *p < 0.05, **p < 0.01 vs. Pb-Mn rats; Group sizes: Total (M/F): Standard Control = 121 (59/62); Standard Mn = 39 (21/18); Standard Pb-Mn = 35 (19/16); Standard Pb = 39 (19/20); Barren Control = 112 (58/54); Barren Mn = 27 (12/15); Barren Pb-Mn = 24 (11/13); Barren Pb = 35 (19/16). Data are LS Mean ± SEM.
For the probe trial 24 h after the last acquisition trial, there were no exposure-related effects for average distance from the platform site, percent time in the target quadrant, or swim speed. Main effects of cage were found for average distance from the platform site (F(1,372) = 6.25, p < 0.01) and percent time in the target quadrant (F(1,372) = 6.00, p < 0.01): Barren-reared rats swam farther from the platform site and spent less time in the target quadrant than Standard-reared rats (Average distance: Barren: 0.064 ± 0.02 m, Standard: 0.058 ± 0.01 m; Percent time: Barren: 42.1 ± 1.4%, Standard: 46.7 ± 1.3%).
Reversal
No main effect of exposure or cage was found for latency, path length, cumulative distance, or swim speed on reversal platform trials. However, exposure × day interactions were found for latency (F(15,1828) = 2.18, p < 0.01), path length (F(15,1832) = 2.08, p < 0.01), and cumulative distance (F(15,1815) = 2.40, p < 0.001). Slice-effect ANOVAs on each day, revealed that Mn and Pb rats had longer latencies, path lengths, and swam farther from the platform than Pb-Mn rats on day 2 (Figure 5E), however this difference was not significant when compared with Controls. Other interactions were exposure × sex × cage for latency (F(3,389) = 2.57, p < 0.05) and path length (F(3,389) = 2.57, p < 0.05) and cage × sex for latency (F(1,393) = 4.59, p < 0.05), path length (F(1,390) = 3.08, p < 0.05), and cumulative distance (F(1,394) = 4.42, p < 0.05). Slice-effect ANOVAs on these interactions revealed no exposure-related effects.
For the reversal probe trial, no exposure- or cage-related effects were found for percent time in the target quadrant. For average distance from the platform site, there was an exposure × sex interaction (F(3,381) = 2.18, p < 0.05), however, post hoc tests failed to show any exposure group being different from Controls.
Shift
There were no main effects of exposure or cage on shift platform trials. For latency and cumulative distance, there were exposure × cage × sex interactions (F(3,378) = 2.61, p < 0.05) and (F(3,377) = 2.10, p < 0.05), respectively. Slice-effect ANOVAs revealed no differences associated with cage condition (not shown). There was an exposure × sex × day interaction for cumulative distance (F(15,1802) = 1.47, p < 0.05), but no exposure differences were found in either sex by slice-effect ANOVA on each sex.
For shift probe, there was no effect of exposure or cage. For percent time in the target quadrant, there was an exposure × cage × sex interaction (F(3,379) = 2.95, p < 0.05), however, slice-effect ANOVAs revealed no differences in males or females within cage condition.
Morris Water Maze Cued
No significant effects of exposure or cage were found for latency on cued-random trials (Exposure: Control: 15.1 ± 0.7 s, Mn: 14.2 ± 1.1 s, Pb: 13.9 ± 1.1 s, Pb-Mn: 16.0 ± 1.2 s; Cage: Barren: 14.6 ± 0.9 s, Standard: 15.0 ± 0.8 s).
Morris Water Maze Cue Rotation
There was a main effect of exposure for latency (F(3,381) = 2.05, p < 0.05), path length (F(3,379) = 2.27, p < 0.05) and cumulative distance (F(3,377) = 2.80, p < 0.05) on platform trials with salient cues rotated; slice-effect ANOVAs, however, revealed no differences among groups (Figure 6A,B). Exposure × sex interactions were also found for latency (F(3,382) = 2.41, p < 0.05), path length (F(3,380) = 3.10, p < 0.05), and cumulative distance (F(3,377) = 2.76, p < 0.05); here again, no exposure-related differences were found by slice-effect ANOVAs done on each sex. Furthermore, there was a cage × day interaction for path length (F(5,1527) = 2.02, p < 0.05); slice-effect ANOVAs revealed that Barren-reared rats had longer path lengths than Standard rats on day 5 (p < 0.01; Figure 6C).
Fig. 6. MWM shift.
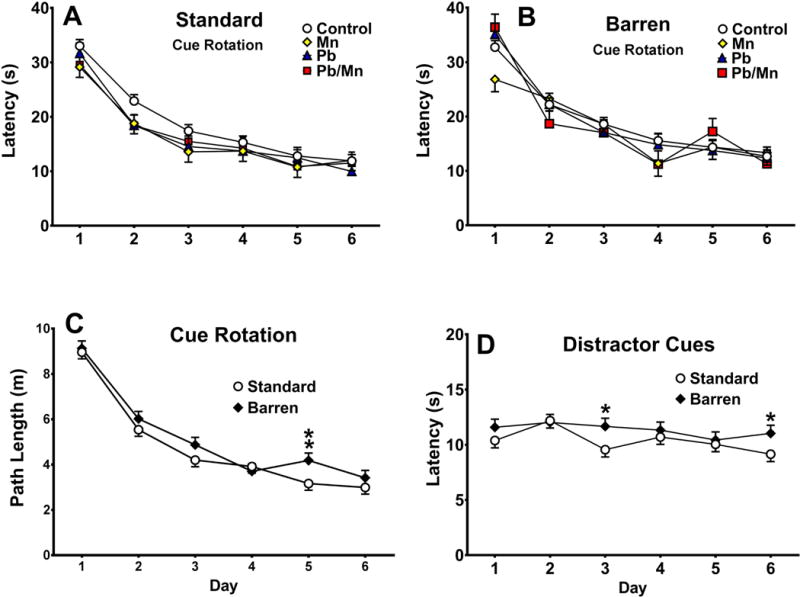
A: Latency in Standard-reared rats; B: Latency Barren-reared rats. Cue Rotation: C: Latency (s) in Standard-reared rats; D: Latency in Barren-reared rats; G: Cage × day interaction for path length (m). Distractor Cues: E: Latency (s) to reach the platform in Standard-reared rats; F: Latency (s) in Barren-reared rats; H: Latency cage × day interaction. Exposure group sizes: Total (M/F): Standard Control = 121 (59/62); Standard Mn = 39 (21/18); Standard Pb-Mn = 35 (19/16); Standard Pb = 39 (19/20); Barren Control = 112 (58/54); Barren Mn = 27 (12/15); Barren Pb-Mn = 24 (11/13); Barren Pb = 35 (19/16). Data are LS Mean ± SEM. *p < 0.05, **p < 0.01 vs. Standard-reared rats.
Morris Water Maze Distractor Cues
For platform trials with distractor cues, exposure effects were found for latency (F(3,370) = 2.42, p < 0.05), path length (F(3,369) = 2.65, p < 0.05) and cumulative distance (F(3,368) = 3.26, p < 0.05); however, slice-effect ANOVAs revealed no differences among exposure groups. Cage × day interactions were found for latency (F(5,1577) = 1.96, p < 0.05) and cumulative distance (F(3,1578) = 1.81, p < 0.05). Post hoc analyses revealed that Barren-reared rats had longer latencies than Standard-reared rats on days 3 and 6 (both p < 0.05, Figure 6D), irrespective of exposure, however, rats were performing optimally at approximately 10 s per trial and therefore no learning curve was seen; this casts doubt on whether the small differences on Day 3 and 6 are meaningful despite being significant.
For the probe trial, there was a main effect of exposure for path length (F(3,316) = 2.12, p < 0.05), but post-hoc tests revealed no significant differences among groups. Exposure × sex interactions were found for path length (F(3,316) = 2.68, p < 0.05) and cumulative distance (F(3,296) = 2.45, p < 0.05); however, slice-effect ANOVAs by sex failed to show exposure differences.
Latent Inhibition
For percent freezing during contextual testing, there was no effect of cage but there was a main effect of exposure (F(3,371) = 3.74, p < 0.01). The Mn and Pb-Mn rats had reduced freezing compared with Controls (p’s < 0.05; Control: 12.50 ± 1.53%, Mn: 6.34 ± 2.50%, Pb: 10.50 ± 2.33%, Pb-Mn: 5.09 ± 2.59%). Also, rats in the PE group spent more time freezing than rats in the NPE group (p < 0.01), but this did not interact with exposure or cage.
For cued conditioning, there was no effect of cage but there was an effect of exposure (F(3,367) = 9.55, p < 0.001); Mn and Pb-Mn rats had reduced freezing to the tone compared with Controls (p < 0.01–0.001; Control: 49.56 ± 2.69 %, Mn: 28.08 ± 4.43 %, Pb: 49.25 ± 4.17 %, Pb-Mn: 34.44 ± 4.59 %). An exposure × sex interaction (F(3,368) = 2.95, p < 0.05) also occurred and revealed that Mn males froze less than males in other groups (p < 0.05–0.001; Figure 7A) and that Mn and Pb-Mn females froze less than Control females (p < 0.05–0.001; Figure 7A). In Standard-reared rats, Mn and Pb-Mn rats had reduced freezing compared with Pb or Control rats (p < 0.05–0.01; Figure 7B). In the Barren group, Mn rats froze less than Pb and Control groups (p < 0.05–0.001; Figure 7B). Rats in the NPE condition (as expected) revealed more freezing to the tone than rats in the PE condition (p < 0.001), and exposure interacted with the PE-NPE factor (F(3,367) = 2.42, p < 0.05). For the NPE condition, Mn and Pb-Mn groups froze less than the Pb and Control groups (p < 0.10–0.01). For the PE condition, Mn rats had less freezing than Pb, Pb-Mn, and Control rats (p < 0.05-0.001) (not shown).
Fig. 7. Latent inhibition.
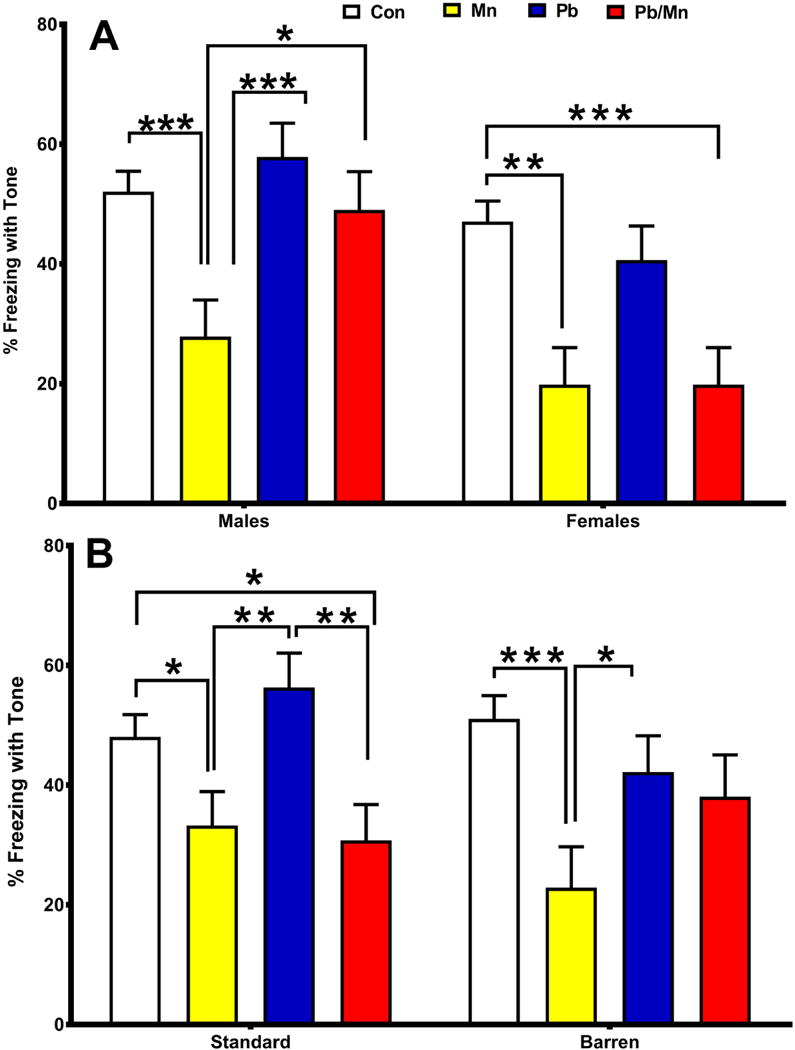
A: There was an exposure × sex interaction for percent (%) freezing with tone during cued conditioning; B: There was also an exposure × cage interaction. Exposure group sizes: Total (M/F): Standard Control = 121 (59/62); Standard Mn = 39 (21/18); Standard Pb-Mn = 35 (19/16); Standard Pb = 39(19/20); Barren Control = 112 (58/54); Barren Mn = 27 (12/15); Barren Pb-Mn = 24 (11/13); Barren Pb = 35 (19/16). Data are LS Mean ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001.
Locomotor Activity after Methamphetamine
Habituation
Rats were re-habituated to the open-field for 30 min prior to methamphetamine administration. There was no effect of exposure or cage during this period, but there was an exposure × time interaction (F(15,1819) = 3.41, p < 0.001); the interaction revealed that Mn and Pb-Mn rats were less active than Controls during the first 5 min and Mn rats were more active than Controls during the last 5 min (all p < 0.01; Figure 8A). There was a cage × sex interaction (p < 0.05), but post hoc tests failed to show a difference between rearing conditions in either sex.
Fig. 8. Open-field with (+)-methamphetamine challenge.
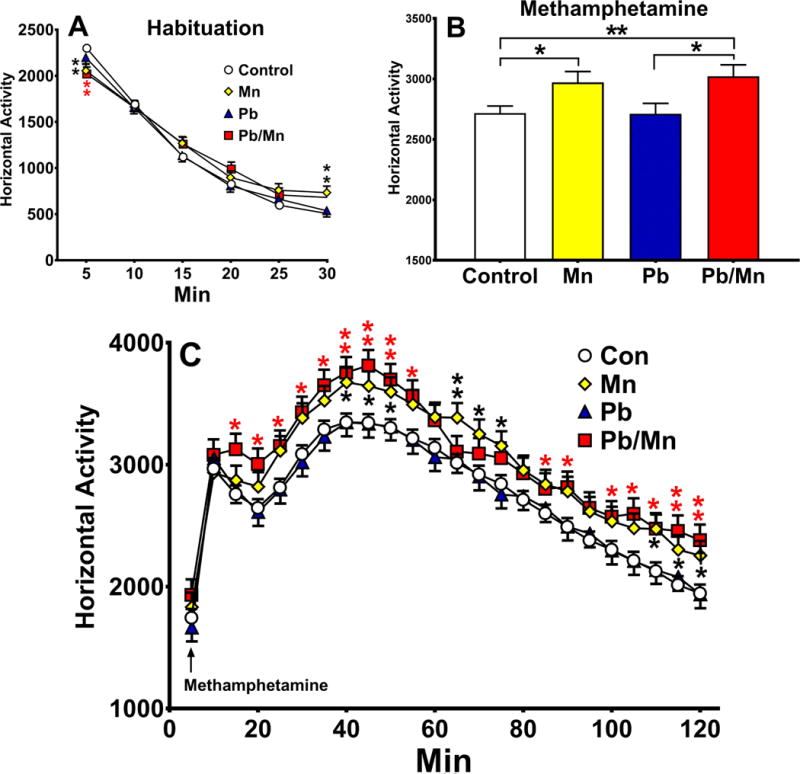
Locomotor activity before and after methamphetamine: A: Exposure × minute interaction prior to methamphetamine administration (habituation); B: Exposure main effect of (+)-methamphetamine averaged across intervals; C: Exposure × minute interaction after (+)-methamphetamine per 5 min interval; Pb-Mn group was more active than Controls during min 10–55, 85–90, and 100–120; the Mn group was more active than Controls during min 20–30, 35–50, 60–75, 110–120. Exposure group sizes: Total (M/F): Control = 221 (112/109); Mn = 64 (32/32); Pb-Mn = 59 (29/30); Pb = 69 (36/33). Data are LS Mean ± SEM. *p < 0.05, **p < 0.01 vs. Controls.
Methamphetamine
Following methamphetamine administration, there was a main effect of methamphetamine at increasing activity, as expected, but relevant to the hypothesis, there as a main effect of exposure (F(3,413) = 5.42, p < 0.001). By post hoc test, the Pb-Mn and Mn rats exhibited greater hyperactivity compared with the Control rats and the Pb-Mn rats compared with Pb rats (p < 0.01; Figure 8B). There was also an exposure × time interaction (F(69,9132) = 1.30, p < 0.05). Slice-effect ANOVAs by time, revealed that the Pb-Mn and Mn groups were more active than Controls during intervals shown in Figure 8C. An exposure × sex × cage × time interaction was also found (F(69,9132) = 1.56, p < 0.01), but slice-effect ANOVAs revealed no exposure differences at any interval as a function of cage or sex.
Table 1 provides a summary of the behavioral effects.
Table 1.
Summary of Results.
| Test | Measure | Pb-Mna | Cageb | Pb-Mn × Cagec |
|---|---|---|---|---|
| EZM | Time in open | Pb-Mn, Mn: ↑ | — | Std Pb ♂: ↓; Std Pb-Mn ♂: ↑; Std Pb-Mn ♀: ↑; Bar Mn ♂: ↑ |
| Light/Dark | Dark entries Latency |
Pb-Mn: ↓ Mn ♂: ↑ |
— Bar ♂: ↓ |
— — |
| OF | Activity | Pb-Mn: ↓ (0-10′), ↑ (40-45′) Mn, Pb: ↓ (0-5′) |
Bar: ↑ (1st 5′) | — |
| PPI | Vmax | Pb-Mn: ↓ Pb-Mn, Mn: ↓ PP-0 Pb-Mn, Mn: ↓ PP-70 vs Pb |
— | — |
| CWM | Errors | Pb-Mn, Mn: ↑ Pb-Mn, Mn ♀: ↑ |
Bar: ↑ | — |
| Latency | Pb-Mn, Mn: ↑ Pb-Mn, Mn ♀: ↑ |
Bar: ↑ | — | |
| MWM Acq | Latency | — | — | Std Mn ♂: ↑ (day 1); Std Pb-Mn ♂: ↑ (day 2) |
| Acq probe | Average distance Quadrant time |
— — |
Bar: ↑ Bar: ↓ |
— — |
| MWM Rev | Latency | Mn, Pb: ↑ (D2) | Bar ♂: ↑ d | — |
| Rev probe | Average distance Quadrant time |
— — |
— — |
— — |
| MWM Shift | Latency | — | — | — |
| Shift probe | Average distance Quadrant time |
— — |
— — |
— — |
| MWM Cued | Latency | — | — | — |
| Cue rotation | Latency | — | — | — |
| Distractors | Latency | — | Bar: ↑ (D3, 6) | — |
| Latent Inhib. | Contextual Cued |
Pb-Mn, Mn: ↓ Pb-Mn, Mn: ↓ Mn ♂: ↓ Pb-Mn, Mn ♀: ↓ |
— — |
— Std Pb-Mn, Mn: ↓ Bar Mn: ↓ |
| OF re-test | Activity | Pb-Mn, Mn: ↓ (1st 5′) Mn: ↑ (last 5′) |
— | — |
| OF Meth | Activity | Pb-Mn, Mn: ↑ | — | — |
Effects in this column indicate exposed vs. Controls unless otherwise specified.
Effects in this column indicate Barren vs. Standard cage.
Effects in this column indicate differences vs. same-sex Controls.
Trend in post hoc test following significant interaction.
Monoamines
Neostriatum
No main effect of exposure or cage was found for DA, DOPAC, or HVA. There were exposure effects for striatal 5-HT (F(3,85.6) = 5.94, p < 0.001) and 5-HIAA (F(3,85.3) = 7.80, p < 0.001). Post-hoc analyses demonstrated that the Pb group had higher 5-HT than the Pb-Mn and Control groups (both p < 0.01; Figure 9A); the Pb group also had higher 5-HIAA compared with all other groups (p < 0.01–0.001; Figure 9B). There was an exposure × cage interaction for 5-HT (F(3,85.6) = 3.13, p < 0.05). Slice-effect ANOVAs revealed that for Standard-reared rats, the Pb group had higher 5-HT levels compared with Controls or other groups (all p < 0.01; Figure 9C); in Barren-reared rats, there were no differences. There were no effects on NE.
Fig. 9. Monoamines.
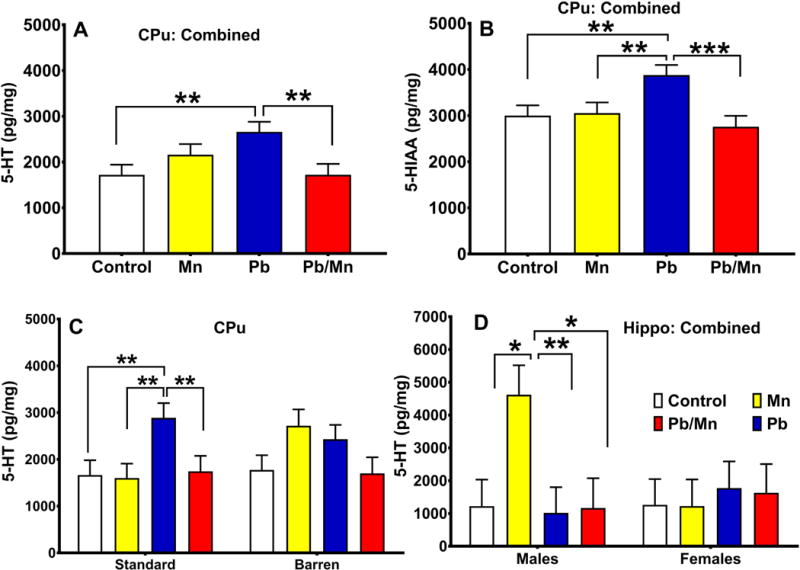
A: 5-HT Effects. There was an exposure main effect for 5-HT in neostriatum (Cpu; cage factor was combined). B: Exposure main effect for 5-HIAA in CPu (cage factor combined). C: There was also an exposure × cage interaction for 5-HT in CPu. D: There was an exposure × sex interaction for 5-HT in hippocampus. Exposure group sizes: Total (M/F): Standard Control = 16 (8/8); Standard Mn = 16 (8/8); Standard Pb-Mn = 13 (6/7); Standard Pb = 15 (8/7); Barren Control = 15 (7/8); Barren Mn = 12 (5/7); Barren Pb-Mn = 12 (6/6); Barren Pb = 16 (8/8). Data are LS Mean ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001.
Hippocampus
There were no main effects of exposure or cage, but there was an exposure × sex interaction (F(3,86.4) = 2.70, p < 0.05) in which Mn males had higher 5-HT levels than Control males or males in other groups (p < 0.01–0.05; Figure 9D); there were no differences in females. There were no effects on 5-HIAA or NE in this region (not shown).
Discussion
Developmental Pb, Mn, and stress exposure are real-world problems (Chiodo et al., 2007; Roels et al., 2012) but their combined effects are unknown. BPb and BMn levels are correlated in children living near industrial plants or urban areas (Carlin et al., 2013; Delves et al., 1973; Sexton et al., 2006; Zielhuis et al., 1978). Chronic stress is a known risk factor by itself (Lupien et al., 2001; McEwen, 1998), and elevated BPb levels and MnOE are reported in low SES areas where children experience elevated stress (Baum et al., 1999; Chiodo et al., 2007). We tested the hypothesis that developmental stress would negatively interact with Pb and Mn exposure to impact behavior and neurochemistry. We used barren cage rearing to model stress because it causes long-term adverse CNS effects (Baum et al., 1999; Gilles et al., 1996).
The data demonstrate that postnatal exposure to Pb, Mn, and Pb-Mn affects behavior long after exposure. Most of the effects were in Mn and Pb-Mn groups, not in the Pb group. The affected groups had decreased anxiety, reduced acoustic startle, impaired egocentric learning, reduced contextual and cued conditioning, reduced MWM acquisition primarily in males, and exaggerated hyperactivity in response to (+)-methamphetamine. MnOE alone increased hippocampal 5-HT in males. Pb alone increased anxiety on the EZM and striatal 5-HT. By itself, barren cage rearing caused deficits in CWM, increased startle, and reduced anxiety-like behavior.
It is difficult to compare the effects in our model versus others for several reasons that include dose, age of administration, route, and outcome measures. For instance, some put metals into the dams’ food or drinking water (Betharia and Maher, 2012; Garcia et al., 2006; Garcia et al., 2007; Kasten-Jolly et al., 2012; Moreira et al., 2001), whereas we administered the metals by gavage in order to control dose (Amos-Kroohs et al., 2016a; Amos-Kroohs et al., 2016b; Graham et al., 2011; Vorhees et al., 2014). Doses were chosen to produce BMn and BPb concentrations similar to those observed in humans (Beaudin et al., 2013; Bellinger, 2008; Haynes et al., 2015; Lanphear et al., 2000; Vorhees et al., 2014). We also previously showed that this Mn dose (100 mg/kg every other day) and exposure period (P4-28) increased neostriatal Mn (VEH = 0.39 ± 0.12 μg/g vs. Mn = 2.39 ± 0.12 μg/g tissue, p ≤ 0.001) and serum Mn levels (i.e., VEH = 11.67 ± 4.75 μg/L vs. Mn = 16.62 ± 4.75 μg/L, p ≤ 0.010) (Amos-Kroohs et al., 2015; Vorhees et al., 2014). These plasma Mn levels are in the range of human exposure (Bhang et al., 2013; Kim et al., 2009; Menezes-Filho et al., 2011). The Pb dose (10 mg/kg every other day from P4-28) previously resulted in a mean BPb level of ~9.0 μg/dL and is in the range of BPb levels found in some children (Amos-Kroohs et al., 2016b; Henn et al., 2012; Lanphear et al., 2005; Rodrigues et al., 2016). The exposure period for both metals was chosen to approximate brain development during late gestation and early childhood (Bayer et al., 1993; Clancy et al., 2007a; Clancy et al., 2007b).
Pb-Mn and Mn reduced anxiety and this is consistent with previous data with the same Mn dose and exposure period (Amos-Kroohs et al., 2015). For other studies assessing developmental MnOE, some find no effects in the elevated plus maze (EPM) (Kern et al., 2010; Pappas et al., 1997), while others find decreased anxiety in the EPM and open-field (Kern et al., 2010; Molina et al., 2011). The latter is more in line with what we see in the Light/Dark test, where Pb-Mn and Mn rats made fewer entries into the dark, and Mn males had longer latencies to enter the dark.
Pb effects on anxiety are mixed. We found that Standard-reared Pb males spent less time in EZM open quadrants, but this was not seen in Barren-reared Pb groups. Others report that Pb from E0-P21 in drinking water reduces exploration in the EPM (Moreira et al., 2001; Trombini et al., 2001). We saw no effects of Pb in the Light/Dark test or on center time in the open-field. Another study found increased anxiety in the open-field following Pb given E8-P21 in drinking water in mice (Kasten-Jolly et al., 2012), whereas still others find no Pb effects in an open-field when Pb is given from E0-P21 in drinking water in rats (Betharia and Maher, 2012). No consistent picture of how developmental Pb affects anxiety in rodents can be ascertained from these divergent results.
MnOE impaired egocentric learning in the CWM, implicating neostriatal pathways (Buzsáki and Moser, 2013). Dopaminergic dysfunction in the striatum is known to impair CWM (Braun et al., 2012; Braun et al., 2015). We reported that developmental MnOE (using the same dosing paradigm as here) alters neostriatal DA and causes CWM deficits (Amos-Kroohs et al., 2016a; Amos-Kroohs et al., 2017) and DA effects were found by others (Kern et al., 2010; McDougall et al., 2008; Moreno et al., 2009). One study showed that MnOE (750 μg, p.o., P1-21) in rats decreased striatal DA transporter (DAT), DA transmission, and reduced performance on a fixed ratio-1 operant task but not on higher ratio schedules of reinforcement (McDougall et al., 2008), further implicating striatal DA, albeit indirectly (McDougall et al., 2008; Vorhees and Williams, 2016). In contrast, Pb-exposure had no effects on CWM. Evidence from others suggest that higher levels of Pb alters DA in the nucleus accumbens (Cory-Slechta, 1997; Cory-Slechta et al., 1997; Cory-Slechta et al., 1998; Widzowski et al., 1994; Zuch et al., 1998), and we found that reduced DA in this region impaired CWM learning, but only if DA reductions are larger than what is reported to occur from Pb exposure (Braun et al., 2016).
Barren-reared rats had more deficits than Standard-reared rats in the CWM. This is consistent with reports that developmental stress causes cognitive deficits (Amos-Kroohs et al., 2017; Champagne et al., 2009; Charil et al., 2010; King and Laplante, 2005; Laplante et al., 2004; Naninck et al., 2015). Stress, however, did not interact with Mn or Pb on CWM outcome (Amos-Kroohs et al., 2017).
Some deficits were found in MWM but only on 1 or 2 days of some phases of the test. Standard-reared males (Mn, Pb, and Pb-Mn) and females (Pb-Mn only) were impaired on 1–2 days, but no effects were seen in Barren-reared Mn or Pb exposed groups. Effects on later phases of the test were not consistent. We previously found that MnOE caused MWM deficits and reduced hippocampal CA1 long-term potentiation (LTP) (Amos-Kroohs et al., 2017), however the MWM was larger in that study than the one used here. The larger tank diameter in the previous study may have made the task sufficiently more difficult that differences were detected that were not seen in the present experiment.
For Pb, there are reports that developmental Pb exposure during gestation and lactation (BPb levels ~35–40 μg/dL) reduced hippocampal neurogenesis but did not impair spatial learning and memory (Gilbert et al., 2005). Others find that Pb given during gestation and lactation causes sex-specific spatial learning deficits in young (Betharia and Maher, 2012; Jett et al., 1997) and old rats (Anderson et al., 2012; Betharia and Maher, 2012) with BPb levels in those studies ranging ~9–55 μg/dL. However, one study reported that perinatal exposure to Pb (BPb ~9 μg/dL) and Mn did not cause MWM impairments in rats (Betharia and Maher, 2012). Studies using different Mn and Pb doses, routes of administration, times of exposure, species, strains, and tests have often found evidence of spatial learning and memory impairment (Betharia and Maher, 2012; Kern et al., 2010; Yang et al., 2003) with BPb levels over 25 μg/dL in the Yang et al. study, while others have not (Gilbert et al., 2005; Pappas et al., 1997). Although the weight of evidence appears to favor Pb having an adverse effect on spatial learning and memory at higher doses in rats, effects at lower doses, including those used in the current study, do not always reproduce those effects.
Barren-cage rearing induced deficits in the MWM regardless of metal exposure. Others also showed that early stress caused impaired hippocampal-related learning (Kapoor et al., 2009; Modir et al., 2014; Naninck et al., 2015) and altered hippocampal neurogenesis (Naninck et al., 2015). Such effects may depend on the type and timing of the stressor, i.e., early vs. late gestation exposure and gestational vs. postnatal exposure (Kapoor et al., 2009; Modir et al., 2014).
Mn and Pb-Mn both decreased contextual freezing; Pb had no effect. During cued recall, Mn and Pb-Mn also impaired memory. Fear conditioning is associated with glutamatergic activity in the hippocampus and amygdala, therefore, the data indirectly implicate that these pathways are affected (Fanselow and Kim, 1994; Gould and Lewis, 2005; Phillips and LeDoux, 1992).
In acoustic startle, Mn and Pb-Mn decreased startle responses, an effect we reported after MnOE (Amos-Kroohs et al., 2016a). One study found increased startle after developmental MnOE at 25 and 50 mg/kg given from P1-21 orally to dams, but the effect was seen only at P21, not later (Dorman et al., 2000). By contrast, another study gave 5, 10, or 20 mg/mL of MnCl2 in drinking water throughout gestation resulting in total intake of 3.7, 6.2, and 9.5 g of MnCl2 and found no differences in acoustic startle responses in the offspring on P13-24 (Kontur and Fechter, 1985).
(+)-Methamphetamine challenge revealed Mn and Pb-Mn rats had exaggerated responses compared with controls; Pb did not alter the typical response. In one of the few cases where co-exposure induced more effects, the hyperactivity of the Pb-Mn group was greater than that in the Mn-only group. Methamphetamine reverses the flux of monoamine reuptake transporters, thereby increasing synaptic neurotransmitter concentrations. Since methamphetamine-induced hyperactivity is largely driven by striatal DA release, our data are consistent with the idea that Mn exposure alters DA function. Developmental MnOE is also known to alter DA receptors (Kern and Smith, 2011; Kern et al., 2010; Tran et al., 2002a; Tran et al., 2002b), and increased extracellular DA was reported in MnOE rodents (Erikson et al., 2005). We previously showed exaggerated hyperactivity after (+)-amphetamine in MnOE rats using the same exposure period used here (Amos-Kroohs et al., 2015). Amphetamine, unlike methamphetamine that affects multiple monoamines, is selective for DA, having little effect on NE or 5-HT. Therefore, taking these two studies together suggests that developmental MnOE alters DA function.
Pb exposure did not alter methamphetamine-induced activity increases. Similarly, no effects of gestational and lactational Pb exposure in drinking water were found in P35 rats after amphetamine challenge (Virgolini et al., 2004). Others report that developmental Pb attenuates amphetamine-induced activity both in adult (Rafales et al., 1981; Reiter et al., 1975) and in P25 rats (Wince et al., 1980).
While Pb did not produce many effects, it did increase neostriatal 5-HT and 5-HIAA levels. Pb did not alter DA, DOPAC, or HVA, although in a previous report Pb increased HVA in neostriatum in Standard-reared rats at younger ages (Graham et al., 2011). Changes in HVA tend to reflect alterations in monoamine oxidase and/or catechol-O-methyltransferase activity; thus, HVA reflects DA utilization (Cooper et al., 2003). Many studies describe effects of developmental stress and Pb on DA in mesocorticolimbic pathways (Cory-Slechta et al., 2004; Rossi-George et al., 2009; Rossi-George et al., 2011; Virgolini et al., 2005; Virgolini et al., 2008) but not in the hippocampus. Consistent with the latter, we found no Pb effects in the hippocampus. We previously found effects on hippocampal 5-HT on P19, during exposure, but the effects depended on dose, sex, and stressor and do not appear to be lasting (Graham et al., 2011).
In conclusion, most of the effects observed in this study were in the Mn and Pb-Mn groups. It is known that Mn uptake is enhanced in young rats due to increased nutritional demand for nutrients during rapid growth (Ballatori et al., 1987; Garcia et al., 2006; Takeda et al., 1999; Yoon et al., 2009). It is possible that Mn given with higher doses of Pb would elicit bigger effects or have more interactions. While the Pb dose used here did not elicit many alterations, studies using higher doses in drinking water report adverse behavioral effects (Cory-Slechta, 2003; Cory-Slechta et al., 2010; Moreira et al., 2001; Rossi-George et al., 2011; Weston et al., 2014). Interestingly, in a study where Mn, Pb, and Pb-Mn were administered to dams in drinking water, there were interactions in which the presence of Pb increased absorption of Mn into brain (Betharia and Maher, 2012). Many of the effects we saw from developmental stress were sex- and context-dependent (Champagne et al., 2009; Cory-Slechta et al., 2010; Cory-Slechta et al., 2012; Kapoor et al., 2009; Luine et al., 2007; Weinstock, 2007), but overall, barren-cage stress had limited interactions with Mn and Pb. Within the confines of this study, Mn had the most consistent and most pronounced effects.
Highlights.
Developmental Mn and Pb-Mn altered anxiety, acoustic startle, open-field, learning and monoamines
Developmental Pb altered anxiety, open-field, and monoamines.
Developmental barren-cage rearing altered anxiety, startle, open-field, and learning.
Few interactions were observed between developmental factors.
Acknowledgments
Supported by NIH grant R01 ES015689 and training grant T32 ES007051.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest:
The authors report no conflict of interest.
References
- Amos-Kroohs RM, et al. Effects of developmental exposure to manganese and/or low iron diet: Changes to metal transporters, sucrose preference, elevated zero-maze, open-field, and locomotion in response to fenfluramine, amphetamine, and MK-801. Toxicol Rep. 2015;2:1046–1056. doi: 10.1016/j.toxrep.2015.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amos-Kroohs RM, et al. Developmental manganese exposure in combination with developmental stress and iron deficiency: Effects on behavior and monoamines. Neurotoxicol Teratol. 2016a;56:55–67. doi: 10.1016/j.ntt.2016.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amos-Kroohs RM, et al. Developmental stress and lead (Pb): Effects of maternal separation and/or Pb on corticosterone, monoamines, and blood Pb in rats. Neurotoxicology. 2016b;54:22–33. doi: 10.1016/j.neuro.2016.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amos-Kroohs RM, et al. Developmental manganese neurotoxicity in rats: Cognitive deficits in allocentric and egocentric learning and memory. Neurotoxicol Teratol. 2017;59:16–26. doi: 10.1016/j.ntt.2016.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson DW, Pothakos K, Schneider JS. Sex and rearing condition modify the effects of perinatal lead exposure on learning and memory. Neurotoxicology. 2012;33:985–95. doi: 10.1016/j.neuro.2012.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anisman H, et al. Do early-life events permanently alter behavioral and hormonal responses to stressors? Int J Dev Neurosci. 1998;16:149–64. doi: 10.1016/s0736-5748(98)00025-2. [DOI] [PubMed] [Google Scholar]
- Aschner JL, Aschner M. Nutritional aspects of manganese homeostasis. Mol Aspects Med. 2005;26:353–62. doi: 10.1016/j.mam.2005.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballatori N, Miles E, Clarkson TW. Homeostatic control of manganese excretion in the neonatal rat. Am J Physiol. 1987;252:R842–7. doi: 10.1152/ajpregu.1987.252.5.R842. [DOI] [PubMed] [Google Scholar]
- Baum A, Garofalo JP, Yali AM. Socioeconomic status and chronic stress. Does stress account for SES effects on health? Ann N Y Acad Sci. 1999;896:131–44. doi: 10.1111/j.1749-6632.1999.tb08111.x. [DOI] [PubMed] [Google Scholar]
- Bayer SA, et al. Timetables of neurogenesis in the human brain based on experimentally determined patterns in the rat. Neurotoxicology. 1993;14:83–144. [PubMed] [Google Scholar]
- Beaudin SA, Nisam S, Smith DR. Early life versus lifelong oral manganese exposure differently impairs skilled forelimb performance in adult rats. Neurotoxicol Teratol. 2013;38:36–45. doi: 10.1016/j.ntt.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellinger DC. Very low lead exposures and children’s neurodevelopment. Curr Opin Pediatr. 2008;20:172–7. doi: 10.1097/MOP.0b013e3282f4f97b. [DOI] [PubMed] [Google Scholar]
- Bellinger DC. The protean toxicities of lead: new chapters in a familiar story. Int J Environ Res Public Health. 2011;8:2593–628. doi: 10.3390/ijerph8072593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betharia S, Maher TJ. Neurobehavioral effects of lead and manganese individually and in combination in developmentally exposed rats. Neurotoxicology. 2012;33:1117–27. doi: 10.1016/j.neuro.2012.06.002. [DOI] [PubMed] [Google Scholar]
- Bhang SY, et al. Relationship between blood manganese levels and children’s attention, cognition, behavior, and academic performance—A nationwide cross-sectional study. Environmental Research. 2013;126:9–16. doi: 10.1016/j.envres.2013.05.006. [DOI] [PubMed] [Google Scholar]
- Bouchard MF, et al. Intellectual impairment in school-age children exposed to manganese from drinking water. Environ Health Perspect. 2011;119:138–43. doi: 10.1289/ehp.1002321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun AA, et al. Comparison of the elevated plus and elevated zero mazes in treated and untreated male Sprague-Dawley rats: effects of anxiolytic and anxiogenic agents. Pharmacol Biochem Behav. 2011;97:406–15. doi: 10.1016/j.pbb.2010.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun AA, et al. Dorsal striatal dopamine depletion impairs both allocentric and egocentric navigation in rats. Neurobiol Learn Mem. 2012;97:402–8. doi: 10.1016/j.nlm.2012.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun AA, et al. Dopamine depletion in either the dorsomedial or dorsolateral striatum impairs egocentric Cincinnati water maze performance while sparing allocentric Morris water maze learning. Neurobiol Learn Mem. 2015;118:55–63. doi: 10.1016/j.nlm.2014.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun AA, et al. -Hydroxydopamine-Induced Dopamine Reductions in the Nucleus Accumbens, but not the Medial Prefrontal Cortex, Impair Cincinnati Water Maze Egocentric and Morris Water Maze Allocentric Navigation in Male Sprague-Dawley Rats. Neurotox Res. 2016:6. doi: 10.1007/s12640-016-9616-6. [DOI] [PubMed] [Google Scholar]
- Burns JM, et al. Lifetime low-level exposure to environmental lead and children’s emotional and behavioral development at ages 11-13 years. The Port Pirie Cohort Study. Am J Epidemiol. 1999;149:740–9. doi: 10.1093/oxfordjournals.aje.a009883. [DOI] [PubMed] [Google Scholar]
- Buzsáki G, Moser EI. Memory, navigation and theta rhythm in the hippocampal-entorhinal system. Nature neuroscience. 2013;16:130–138. doi: 10.1038/nn.3304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canfield RL, et al. Intellectual impairment in children with blood lead concentrations below 10 microg per deciliter. N Engl J Med. 2003;348:1517–26. doi: 10.1056/NEJMoa022848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlin DJ, et al. Unraveling the Health Effects of Environmental Mixtures: An NIEHS Priority. Environmental Health Perspectives. 2013;121:a6–a8. doi: 10.1289/ehp.1206182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cecil KM, et al. Decreased brain volume in adults with childhood lead exposure. PLoS Med. 2008;5:e112. doi: 10.1371/journal.pmed.0050112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S.D.o.H.a.H. Services, editor. Centers for Disease Control and Prevention. Preventing Lead Poisoning in Young Children: A Statement of the Centers for Disease Control and Prevention. Atlanta, GA: 2005. pp. 1–137. [Google Scholar]
- D.o.E.a.E.H.S. National Cetner for Environmental Health, editor. Centers for Disease Control and Prevention. CDC’s Childhood Lead Poisoning Prevention Program: What do Parents Need to Know to Protect Their Children? Atlanta, GA: 2012. [Google Scholar]
- Champagne DL, Ronald de Kloet E, Joëls M. Fundamental aspects of the impact of glucocorticoids on the (immature) brain. Seminars in Fetal and Neonatal Medicine. 2009;14:136–142. doi: 10.1016/j.siny.2008.11.006. [DOI] [PubMed] [Google Scholar]
- Charil A, et al. Prenatal stress and brain development. Brain Research Reviews. 2010;65:56–79. doi: 10.1016/j.brainresrev.2010.06.002. [DOI] [PubMed] [Google Scholar]
- Chiodo LM, et al. Blood lead levels and specific attention effects in young children. Neurotoxicol Teratol. 2007;29:538–46. doi: 10.1016/j.ntt.2007.04.001. [DOI] [PubMed] [Google Scholar]
- Clancy B, Darlington RB, Finlay BL. Translating developmental time across mammalian species. Neuroscience. 2001;105:7–17. doi: 10.1016/s0306-4522(01)00171-3. [DOI] [PubMed] [Google Scholar]
- Clancy B, et al. Extrapolating brain development from experimental species to humans. Neurotoxicology. 2007a;28:931–7. doi: 10.1016/j.neuro.2007.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clancy B, et al. Web-based method for translating neurodevelopment from laboratory species to humans. Neuroinformatics. 2007b;5:79–94. doi: 10.1385/ni:5:1:79. [DOI] [PubMed] [Google Scholar]
- Cohen S, Doyle WJ, Baum A. Socioeconomic status is associated with stress hormones. Psychosom Med. 2006;68:414–20. doi: 10.1097/01.psy.0000221236.37158.b9. [DOI] [PubMed] [Google Scholar]
- Collipp PJ, Chen SY, Maitinsky S. Manganese in infant formulas and learning disability. Ann Nutr Metab. 1983;27:488–94. doi: 10.1159/000176724. [DOI] [PubMed] [Google Scholar]
- Cooper JR, Bloom FE, Roth RH. The Biochemical Basis of Neuropharmacology. Vol. 1. Oxford University Press; New York: 2003. [Google Scholar]
- Cory-Slechta DA. Relationships between Pb-induced changes in neurotransmitter system function and behavioral toxicity. Neurotoxicology. 1997;18:673–88. [PubMed] [Google Scholar]
- Cory-Slechta DA, Pazmino R, Bare C. The critical role of nucleus accumbens dopamine systems in the mediation of fixed interval schedule-controlled operant behavior. Brain Research. 1997;764:253–256. doi: 10.1016/s0006-8993(97)00591-x. [DOI] [PubMed] [Google Scholar]
- Cory-Slechta DA, O’Mara DJ, Brockel BJ. Nucleus Accumbens Dopaminergic Medication of Fixed Interval Schedule-Controlled Behavior and Its Modulation by Low-Level Lead Exposure. Journal of Pharmacology and Experimental Therapeutics. 1998;286:794. [PubMed] [Google Scholar]
- Cory-Slechta DA. Lead-induced impairments in complex cognitive function: offerings from experimental studies. Child Neuropsychol. 2003;9:54–75. doi: 10.1076/chin.9.1.54.14499. [DOI] [PubMed] [Google Scholar]
- Cory-Slechta DA, et al. Maternal stress modulates the effects of developmental lead exposure. Environ Health Perspect. 2004;112:717–30. doi: 10.1289/ehp.6481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cory-Slechta DA, et al. Enhanced learning deficits in female rats following lifetime pb exposure combined with prenatal stress. Toxicol Sci. 2010;117:427–38. doi: 10.1093/toxsci/kfq221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cory-Slechta DA, et al. Enhanced stimulus sequence-dependent repeated learning in male offspring after prenatal stress alone or in conjunction with lead exposure. Neurotoxicology. 2012;33:1188–202. doi: 10.1016/j.neuro.2012.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cory-Slechta DA, et al. Variations in the nature of behavioral experience can differentially alter the consequences of developmental exposures to lead, prenatal stress, and the combination. Toxicol Sci. 2013a;131:194–205. doi: 10.1093/toxsci/kfs260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cory-Slechta DA, et al. Brain hemispheric differences in the neurochemical effects of lead, prenatal stress, and the combination and their amelioration by behavioral experience. Toxicol Sci. 2013b;132:419–30. doi: 10.1093/toxsci/kft015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Kloet ER, et al. Stress, glucocorticoids and development. Prog Brain Res. 1988;73:101–20. doi: 10.1016/S0079-6123(08)60500-2. [DOI] [PubMed] [Google Scholar]
- Delves HT, Clayton BE, Bicknell J. Concentration of trace metals in the blood of children. Br J Prev Soc Med. 1973;27:100–7. doi: 10.1136/jech.27.2.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich KN, et al. Early exposure to lead and juvenile delinquency. Neurotoxicol Teratol. 2001;23:511–8. doi: 10.1016/s0892-0362(01)00184-2. [DOI] [PubMed] [Google Scholar]
- Dorman DC, et al. Neurotoxicity of manganese chloride in neonatal and adult CD rats following subchronic (21-day) high-dose oral exposure. J Appl Toxicol. 2000;20:179–87. doi: 10.1002/(sici)1099-1263(200005/06)20:3<179::aid-jat631>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Erikson KM, et al. Manganese accumulation in striatum of mice exposed to toxic doses is dependent upon a functional dopamine transporter. Environ Toxicol Pharmacol. 2005;20:390–4. doi: 10.1016/j.etap.2005.03.009. [DOI] [PubMed] [Google Scholar]
- Fanselow MS, Kim JJ. Acquisition of contextual Pavlovian fear conditioning is blocked by application of an NMDA receptor antagonist D, L-2-amino-5-phosphonovaleric acid to the basolateral amygdala. Behav Neurosci. 1994;108:210–2. doi: 10.1037//0735-7044.108.1.210. [DOI] [PubMed] [Google Scholar]
- Garcia SJ, et al. A manganese-enhanced diet alters brain metals and transporters in the developing rat. Toxicol Sci. 2006;92:516–25. doi: 10.1093/toxsci/kfl017. [DOI] [PubMed] [Google Scholar]
- Garcia SJ, et al. Iron deficient and manganese supplemented diets alter metals and transporters in the developing rat brain. Toxicol Sci. 2007;95:205–14. doi: 10.1093/toxsci/kfl139. [DOI] [PubMed] [Google Scholar]
- Gilbert ME, et al. Chronic Developmental Lead Exposure Reduces Neurogenesis in Adult Rat Hippocampus but Does Not Impair Spatial Learning. Toxicological Sciences. 2005;86:365–374. doi: 10.1093/toxsci/kfi156. [DOI] [PubMed] [Google Scholar]
- Gilles EE, Schultz L, Baram TZ. Abnormal corticosterone regulation in an immature rat model of continuous chronic stress. Pediatr Neurol. 1996;15:114–9. doi: 10.1016/0887-8994(96)00153-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gos T, et al. Stress-induced synaptic changes in the rat anterior cingulate cortex are dependent on endocrine developmental time windows. Synapse. 2008;62:229–232. doi: 10.1002/syn.20477. [DOI] [PubMed] [Google Scholar]
- Gould TJ, Lewis MC. Coantagonism of glutamate receptors and nicotinic acetylcholinergic receptors disrupts fear conditioning and latent inhibition of fear conditioning. Learn Mem. 2005;12:389–98. doi: 10.1101/lm.89105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham DL, et al. Effects of developmental stress and lead (Pb) on corticosterone after chronic and acute stress, brain monoamines, and blood Pb levels in rats. Int J Dev Neurosci. 2011;29:45–55. doi: 10.1016/j.ijdevneu.2010.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruss M, et al. Maternal separation during a specific postnatal time window prevents reinforcement of hippocampal long-term potentiation in adolescent rats. Neuroscience. 2008;152:1–7. doi: 10.1016/j.neuroscience.2007.12.033. [DOI] [PubMed] [Google Scholar]
- Gump BB, et al. Prenatal and early childhood blood lead levels and cardiovascular functioning in 9(1/2) year old children. Neurotoxicol Teratol. 2005;27:655–65. doi: 10.1016/j.ntt.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Gump BB, et al. Low-level prenatal and postnatal blood lead exposure and adrenocortical responses to acute stress in children. Environ Health Perspect. 2008;116:249–55. doi: 10.1289/ehp.10391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes EN, et al. Manganese Exposure and Neurocognitive Outcomes in Rural School-Age Children: The Communities Actively Researching Exposure Study (Ohio, USA) Environ Health Perspect. 2015;123:1066–71. doi: 10.1289/ehp.1408993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henn BC, et al. Associations of Early Childhood Manganese and Lead Coexposure with Neurodevelopment. Environmental Health Perspectives. 2012;120:126–131. doi: 10.1289/ehp.1003300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henn BC, Coull BA, Wright RO. Chemical Mixtures and Children’s Health. Current opinion in pediatrics. 2014;26:223–229. doi: 10.1097/MOP.0000000000000067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu H, Shine J, Wright RO. The challenge posed to children’s health by mixtures of toxic waste: the Tar Creek Superfund Site as a case-study. Pediatric clinics of North America. 2007;54:155–x. doi: 10.1016/j.pcl.2006.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivy AS, et al. Dysfunctional nurturing behavior in rat dams with limited access to nesting material: a clinically relevant model for early-life stress. Neuroscience. 2008;154:1132–42. doi: 10.1016/j.neuroscience.2008.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs DE, et al. The prevalence of lead-based paint hazards in U.S. housing. Environ Health Perspect. 2002;110:A599–606. doi: 10.1289/ehp.021100599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jett DA, et al. Age-Dependent Effects of Developmental Lead Exposure on Performance in the Morris Water Maze. Pharmacology Biochemistry and Behavior. 1997;57:271–279. doi: 10.1016/s0091-3057(96)00350-4. [DOI] [PubMed] [Google Scholar]
- Joselow MM, et al. Manganese pollution in the city environment and its relationship to traffic density. Am J Public Health. 1978;68:557–60. doi: 10.2105/ajph.68.6.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapoor A, et al. The effects of prenatal stress on learning in adult offspring is dependent on the timing of the stressor. Behavioural Brain Research. 2009;197:144–149. doi: 10.1016/j.bbr.2008.08.018. [DOI] [PubMed] [Google Scholar]
- Kasten-Jolly J, et al. Developmental lead effects on behavior and brain gene expression in male and female BALB/cAnNTac mice. Neurotoxicology. 2012;33:1005–20. doi: 10.1016/j.neuro.2012.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern C, Smith DR. Pre-weaning Mn exposure leads to prolonged astrocyte activation and lasting effects on the dopaminergic system in adult male rats. Vol. 65. Synapse; New York, N.Y.: 2011. pp. 532–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern CH, Stanwood GD, Smith DR. Preweaning manganese exposure causes hyperactivity, disinhibition, and spatial learning and memory deficits associated with altered dopamine receptor and transporter levels. Synapse. 2010;64:363–78. doi: 10.1002/syn.20736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan K, et al. Manganese exposure from drinking water and children’s classroom behavior in Bangladesh. Environ Health Perspect. 2011;119:1501–6. doi: 10.1289/ehp.1003397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan K, et al. Manganese exposure from drinking water and children’s academic achievement. Neurotoxicology. 2012;33:91–7. doi: 10.1016/j.neuro.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y, et al. Co-exposure to environmental lead and manganese affects the intelligence of school-aged children. NeuroToxicology. 2009;30:564–571. doi: 10.1016/j.neuro.2009.03.012. [DOI] [PubMed] [Google Scholar]
- King S, Laplante DP. The effects of prenatal maternal stress on children’s cognitive development: Project Ice Storm. Stress. 2005;8:35–45. doi: 10.1080/10253890500108391. [DOI] [PubMed] [Google Scholar]
- Kontur PJ, Fechter LD. Brain manganese, catecholamine turnover, and the development of startle in rats prenatally exposed to manganese. Teratology. 1985;32:1–11. doi: 10.1002/tera.1420320102. [DOI] [PubMed] [Google Scholar]
- Lanphear BP, et al. Cognitive deficits associated with blood lead concentrations <10 microg/dL in US children and adolescents. Public Health Rep. 2000;115:521–9. doi: 10.1093/phr/115.6.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanphear BP, et al. Low-level environmental lead exposure and children’s intellectual function: an international pooled analysis. Environ Health Perspect. 2005;113:894–9. doi: 10.1289/ehp.7688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laplante DP, et al. Stress During Pregnancy Affects General Intellectual and Language Functioning in Human Toddlers. Pediatr Res. 2004;56:400–410. doi: 10.1203/01.PDR.0000136281.34035.44. [DOI] [PubMed] [Google Scholar]
- Levin R, et al. Lead exposures in U.S. Children, 2008: implications for prevention. Environ Health Perspect. 2008;116:1285–93. doi: 10.1289/ehp.11241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonnerdal B. Nutritional aspects of soy formula. Acta Paediatr Suppl. 1994;402:105–8. doi: 10.1111/j.1651-2227.1994.tb13371.x. [DOI] [PubMed] [Google Scholar]
- Lucchini RG, et al. Tremor, olfactory and motor changes in Italian adolescents exposed to historical ferro-manganese emission. Neurotoxicology. 2012;33:687–96. doi: 10.1016/j.neuro.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luine VN, et al. Chronic Stress and Neural Function: Accounting for Sex and Age. Journal of Neuroendocrinology. 2007;19:743–751. doi: 10.1111/j.1365-2826.2007.01594.x. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, et al. Increased cortisol levels and impaired cognition in human aging: implication for depression and dementia in later life. Rev Neurosci. 1999;10:117–39. doi: 10.1515/revneuro.1999.10.2.117. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, et al. Child’s stress hormone levels correlate with mother’s socioeconomic status and depressive state. Biol Psychiatry. 2000;48:976–80. doi: 10.1016/s0006-3223(00)00965-3. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, et al. Can poverty get under your skin? basal cortisol levels and cognitive function in children from low and high socioeconomic status. Dev Psychopathol. 2001;13:653–76. doi: 10.1017/s0954579401003133. [DOI] [PubMed] [Google Scholar]
- McDougall SA, et al. Postnatal Manganese Exposure Alters Dopamine Transporter Function in Adult Rats: Potential Impact on Nonassociative and Associative Processes. Neuroscience. 2008;154:848–860. doi: 10.1016/j.neuroscience.2008.03.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, et al. Paradoxical effects of adrenal steroids on the brain: protection versus degeneration. Biol Psychiatry. 1992;31:177–99. doi: 10.1016/0006-3223(92)90204-d. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Stellar E. Stress and the individual. Mechanisms leading to disease. Arch Intern Med. 1993;153:2093–101. [PubMed] [Google Scholar]
- McEwen BS. Stress, adaptation, and disease. Allostasis and allostatic load. Ann N Y Acad Sci. 1998;840:33–44. doi: 10.1111/j.1749-6632.1998.tb09546.x. [DOI] [PubMed] [Google Scholar]
- Mendez-Gomez J, et al. Genotoxic effects of environmental exposure to arsenic and lead on children in region Lagunera, Mexico. Ann N Y Acad Sci. 2008;1140:358–67. doi: 10.1196/annals.1454.027. [DOI] [PubMed] [Google Scholar]
- Menezes-Filho JA, et al. Elevated manganese and cognitive performance in school-aged children and their mothers. Environmental research. 2011;111:156–163. doi: 10.1016/j.envres.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modir F, et al. Prenatal stress decreases spatial learning and memory retrieval of the adult male offspring of rats. Physiology & Behavior. 2014;129:104–109. doi: 10.1016/j.physbeh.2014.02.040. [DOI] [PubMed] [Google Scholar]
- Molina RM, et al. Ingestion of Mn and Pb by rats during and after pregnancy alters iron metabolism and behavior in offspring. Neurotoxicology. 2011;32:413–22. doi: 10.1016/j.neuro.2011.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreira EG, Vassilieff I, Vassilieff VSl. Developmental lead exposure: behavioral alterations in the short and long term. Neurotoxicology and Teratology. 2001;23:489–495. doi: 10.1016/s0892-0362(01)00159-3. [DOI] [PubMed] [Google Scholar]
- Moreno JA, et al. Age-Dependent Susceptibility to Manganese-Induced Neurological Dysfunction. Toxicological Sciences. 2009;112:394–404. doi: 10.1093/toxsci/kfp220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muntner P, et al. Continued decline in blood lead levels among adults in the United States: the National Health and Nutrition Examination Surveys. Arch Intern Med. 2005;165:2155–61. doi: 10.1001/archinte.165.18.2155. [DOI] [PubMed] [Google Scholar]
- Naess O, et al. Air pollution, social deprivation, and mortality: a multilevel cohort study. Epidemiology. 2007;18:686–94. doi: 10.1097/EDE.0b013e3181567d14. [DOI] [PubMed] [Google Scholar]
- Naninck EFG, et al. Chronic early life stress alters developmental and adult neurogenesis and impairs cognitive function in mice. Hippocampus. 2015;25:309–328. doi: 10.1002/hipo.22374. [DOI] [PubMed] [Google Scholar]
- Needleman HL, et al. Deficits in psychologic and classroom performance of children with elevated dentine lead levels. N Engl J Med. 1979;300:689–95. doi: 10.1056/NEJM197903293001301. [DOI] [PubMed] [Google Scholar]
- Nigg JT, et al. Low blood lead levels associated with clinically diagnosed attention-deficit/hyperactivity disorder and mediated by weak cognitive control. Biol Psychiatry. 2008;63:325–31. doi: 10.1016/j.biopsych.2007.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappas BA, et al. Perinatal manganese exposure: behavioral, neurochemical, and histopathological effects in the rat. Neurotoxicol Teratol. 1997;19:17–25. doi: 10.1016/s0892-0362(96)00185-7. [DOI] [PubMed] [Google Scholar]
- Patel A, Athawale A. Blood lead levels in children with encephalopathy. Indian Pediatr. 2009;46:845–848. [PubMed] [Google Scholar]
- Phillips RG, LeDoux JE. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behav Neurosci. 1992;106:274–85. doi: 10.1037//0735-7044.106.2.274. [DOI] [PubMed] [Google Scholar]
- Racette BA, et al. Pathophysiology of manganese-associated neurotoxicity. Neurotoxicology. 2012;33:881–6. doi: 10.1016/j.neuro.2011.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rafales LS, et al. Responsiveness to d-amphetamine in lead-exposed rats as measured by steady state levels of catecholamines and locomotor activity. Neurobehav Toxicol Teratol. 1981;3:363–7. [PubMed] [Google Scholar]
- Reiter LW, et al. Developmental and behavioral changes in the rat during chronic exposure to lead. Environmental Health Perspectives. 1975;12:119–123. doi: 10.1289/ehp.7512119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice CJ, et al. A novel mouse model for acute and long-lasting consequences of early life stress. Endocrinology. 2008;149:4892–900. doi: 10.1210/en.2008-0633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues EG, et al. Neurodevelopmental outcomes among 2- to 3-year-old children in Bangladesh with elevated blood lead and exposure to arsenic and manganese in drinking water. Environ Health. 2016;15:44. doi: 10.1186/s12940-016-0127-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roels HA, et al. Manganese exposure and cognitive deficits: A growing concern for manganese neurotoxicity. NeuroToxicology. 2012;33:872–880. doi: 10.1016/j.neuro.2012.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi-George A, et al. Alterations in glucocorticoid negative feedback following maternal Pb, prenatal stress and the combination: A potential biological unifying mechanism for their corresponding disease profiles. Toxicology and Applied Pharmacology. 2009;234:117–127. doi: 10.1016/j.taap.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi-George A, et al. Interactions of lifetime lead exposure and stress: Behavioral, neurochemical and HPA axis effects. NeuroToxicology. 2011;32:83–99. doi: 10.1016/j.neuro.2010.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth JA. Are there common biochemical and molecular mechanisms controlling manganism and parkisonism. Neuromolecular Med. 2009;11:281–96. doi: 10.1007/s12017-009-8088-8. [DOI] [PubMed] [Google Scholar]
- Sanders AP, Claus Henn B, Wright RO. Perinatal and Childhood Exposure to Cadmium, Manganese, and Metal Mixtures and Effects on Cognition and Behavior: A Review of Recent Literature. Curr Environ Health Rep. 2015;2:284–94. doi: 10.1007/s40572-015-0058-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapolsky RM, Meaney MJ. Maturation of the adrenocortical stress response: neuroendocrine control mechanisms and the stress hyporesponsive period. Brain Res. 1986;396:64–76. doi: 10.1016/s0006-8993(86)80190-1. [DOI] [PubMed] [Google Scholar]
- Schnaas L, et al. Blood lead secular trend in a cohort of children in Mexico City (1987-2002) Environ Health Perspect. 2004;112:1110–5. doi: 10.1289/ehp.6636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sexton K, et al. Using Biologic Markers in Blood to Assess Exposure to Multiple Environmental Chemicals for Inner-City Children 3-6 Years of Age. Environmental Health Perspectives. 2006;114:453–459. doi: 10.1289/ehp.8324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surkan PJ, et al. Neuropsychological function in children with blood lead levels <10 microg/dL. Neurotoxicology. 2007;28:1170–7. doi: 10.1016/j.neuro.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda A, Ishiwatari S, Okada S. Manganese uptake into rat brain during development and aging. J Neurosci Res. 1999;56:93–8. doi: 10.1002/(SICI)1097-4547(19990401)56:1<93::AID-JNR12>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Tran TT, et al. Effect of high dietary manganese intake of neonatal rats on tissue mineral accumulation, striatal dopamine levels, and neurodevelopmental status. Neurotoxicology. 2002a;23:635–43. doi: 10.1016/s0161-813x(02)00091-8. [DOI] [PubMed] [Google Scholar]
- Tran TT, et al. Effects of neonatal dietary manganese exposure on brain dopamine levels and neurocognitive functions. Neurotoxicology. 2002b;23:645–51. doi: 10.1016/s0161-813x(02)00068-2. [DOI] [PubMed] [Google Scholar]
- Trombini TV, et al. Developmental lead exposure in rats: is a behavioral sequel extended at F2 generation? Pharmacology Biochemistry and Behavior. 2001;68:743–751. doi: 10.1016/s0091-3057(01)00473-7. [DOI] [PubMed] [Google Scholar]
- Virgolini MB, et al. Amphetamine and stress responses in developmentally lead-exposed rats. Neurotoxicology and Teratology. 2004;26:291–303. doi: 10.1016/j.ntt.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Virgolini MB, et al. Interactions of chronic lead exposure and intermittent stress: consequences for brain catecholamine systems and associated behaviors and HPA axis function. Toxicol Sci. 2005;87:469–82. doi: 10.1093/toxsci/kfi269. [DOI] [PubMed] [Google Scholar]
- Virgolini MB, et al. CNS effects of developmental Pb exposure are enhanced by combined maternal and offspring stress. Neurotoxicology. 2008;29:812–27. doi: 10.1016/j.neuro.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorhees CV. Maze learning in rats: a comparison of performance in two water mazes in progeny prenatally exposed to different doses of phenytoin. Neurotoxicol Teratol. 1987;9:235–41. doi: 10.1016/0892-0362(87)90008-0. [DOI] [PubMed] [Google Scholar]
- Vorhees CV, Williams MT. Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nat Protoc. 2006;1:848–58. doi: 10.1038/nprot.2006.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorhees CV, et al. Effects of neonatal (+)-methamphetamine on path integration and spatial learning in rats: effects of dose and rearing conditions. Int J Dev Neurosci. 2008;26:599–610. doi: 10.1016/j.ijdevneu.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorhees CV, et al. Effects of developmental manganese, stress, and the combination of both on monoamines, growth, and corticosterone. Toxicology reports. 2014;1:1046–1061. doi: 10.1016/j.toxrep.2014.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorhees CV, Williams MT. Cincinnati water maze: A review of the development, methods, and evidence as a test of egocentric learning and memory. Neurotoxicol Teratol. 2016;57:1–19. doi: 10.1016/j.ntt.2016.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, et al. Study of lead absorption and its effect on children’s development. Biomed Environ Sci. 1989;2:325–30. [PubMed] [Google Scholar]
- Weinstock M. Gender differences in the effects of prenatal stress on brain development and behaviour. Neurochem Res. 2007;32:1730–40. doi: 10.1007/s11064-007-9339-4. [DOI] [PubMed] [Google Scholar]
- Weston HI, et al. Sex-dependent impacts of low-level lead exposure and prenatal stress on impulsive choice behavior and associated biochemical and neurochemical manifestations. NeuroToxicology. 2014;44:169–183. doi: 10.1016/j.neuro.2014.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widzowski DV, et al. Time course of postnatal lead-induced changes in dopamine receptors and their relationship to changes in dopamine sensitivity. Neurotoxicology. 1994;15:853–65. [PubMed] [Google Scholar]
- Wince LC, Donovan CA, Azzaro AJ. Alterations in the biochemical properties of central dopamine synapses following chronic postnatal PbCO3 exposure. Journal of Pharmacology and Experimental Therapeutics. 1980;214:642. [PubMed] [Google Scholar]
- Wright JP, et al. Association of prenatal and childhood blood lead concentrations with criminal arrests in early adulthood. PLoS Med. 2008;5:e101. doi: 10.1371/journal.pmed.0050101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, et al. Lead exposure through gestation-only caused long-term learning/memory deficits in young adult offspring. Experimental Neurology. 2003;184:489–495. doi: 10.1016/s0014-4886(03)00272-3. [DOI] [PubMed] [Google Scholar]
- Yoon M, et al. Lactational transfer of manganese in rats: predicting manganese tissue concentration in the dam and pups from inhalation exposure with a pharmacokinetic model. Toxicol Sci. 2009;112:23–43. doi: 10.1093/toxsci/kfp197. [DOI] [PubMed] [Google Scholar]
- Yuan W, et al. The impact of early childhood lead exposure on brain organization: a functional magnetic resonance imaging study of language function. Pediatrics. 2006;118:971–7. doi: 10.1542/peds.2006-0467. [DOI] [PubMed] [Google Scholar]
- Zielhuis RL, et al. Levels of lead and other metals in human blood: suggestive relationships, determining factors. Environ Health Perspect. 1978;25:103–9. doi: 10.1289/ehp.7825103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuch CL, O’Mara DJ, Cory-Slechta DA. Low-Level Lead Exposure Selectively Enhances Dopamine Overflow in Nucleus Accumbens: AnIn VivoElectrochemistry Time Course Assessment. Toxicology and Applied Pharmacology. 1998;150:174–185. doi: 10.1006/taap.1998.8396. [DOI] [PubMed] [Google Scholar]


