Abstract
Background
Evidence suggests both that chronic inflammation mediates the association of food insecurity with adverse health outcomes and that diet may be a significant source of inflammation among food insecure individuals.
Objective
To examine whether food security status is associated with dietary inflammatory potential.
Design & Participants
Cross-sectional data came from the National Health and Nutrition Examination Survey (NHANES), cycles 2007–2014 (n=10,630). The analysis sample is representative of non-institutionalized United States (US) adults with an income-to-poverty ratio ≤3.00.
Main Outcome
Dietary Inflammatory Index (DII) score, calculated using the average of two 24 hour dietary recalls was the main outcome measure.
Statistical analysis
Type III F-tests or χ2 tests compared population characteristics by food security status, defined using the US Food Security Survey Module. Multivariable linear regression was used to estimate the association between food security status and the DII score and moderation by demographic factors. Survey weighting procedures accounted for the effects of stratification and clustering used in the NHANES study design.
Results
When accounting for socioeconomic status, demographic factors, and health status, DII score was higher at greater levels of food insecurity (p-value = 0.0033). Those with very low food security had a 0.31 (95% CI=0.12 to 0.49) higher DII score than those with high food security. Age moderated the association between food security status and DII score (interaction p-value = 0.0103), where the magnitude of the association between DII score and severity of food insecurity was higher for those ≥65 years than for younger age groups.
Conclusion
Food security status may be associated with dietary inflammatory potential, which is hypothesized to play a role in multiple chronic health conditions. Further research is needed to determine the causal nature of this relationship and evaluate how best to implement programs designed to address health disparities within food insecure populations.
Keywords: dietary inflammatory potential, food insecurity, social determinants of health, chronic systemic inflammation, socioeconomic health disparities
Background
Food insecurity, defined as a lack of access to food of sufficient quality or quantity due to financial constraints,1 affects 14% of the United States (US) population.2 Food insecurity is associated with numerous negative health outcomes among adults including a host of complex chronic conditions such as heart disease, diabetes, hypertension, hyperlipidemia, and poor mental health and depression.3–5 However, the underlying biological mechanisms and pathways by which food insecurity influences health are not well understood.
Immune-inflammatory pathways represent a potential link between food insecurity and multiple chronic diseases. Inflammation is an important element of the immune response and necessary to protect against external pathogens and repair tissue damaged by infection or trauma.6 However, certain factors can lead to the development of systemic inflammation, which is considered important in the development of cancer,7 cardiovascular disease,8 and type II diabetes.9 Compelling evidence suggests that systemic inflammation may also result in neurodegenerative and neuropsychiatric illnesses.7,10,11 Thus, identifying socio-environmental or behavioral factors such as food insecurity that contribute to the activation of systemic inflammation and the production of inflammatory cytokines may inform novel intervention strategies.
Multiple aspects of food insecurity have the potential to result in systemic inflammation. The psychological stress and emotional strain associated with experiencing food insecurity enhances the innate immune response and increases production of pro-inflammatory cytokines.12 Additionally, food insecurity is believed to increase the risk for obesity since nutrient-poor, calorically dense foods are often more affordable than healthier options;13 and, inflammatory markers present in adipose tissue can spread to the rest of the body.14 Furthermore, diet itself may be an important source of systemic inflammation.
Nutrients obtained through diet, such as flavonoids, zinc, and n-6 fatty acids, have anti-inflammatory properties shown to reduce most chronic disease risk.15–17 In addition, diets rich in saturated fats and low in fruits and vegetables are believed to be pro-inflammatory compared to diets with fewer saturated fats, and high in fiber rich foods including fruits and vegetables.18,19 It is well established that food insecure individuals consume fewer fruits, vegetables, and dairy products and have significantly lower intake of vitamins A and B6, calcium, magnesium, and zinc.20 Despite this supporting evidence, limited research has directly evaluated whether food insecurity is predictive of overall dietary inflammatory potential or examined the role dietary inflammatory potential may play in mediating the relationship between food insecurity and adverse health outcomes.
A novel strategy to quantify the total inflammatory potential of diet has been developed for use across multiple studies of diet and health.21,22 Originally produced by Cavicchia et al. (2009) and refined by Shivappa et al. (2014), the Dietary Inflammatory Index (DII) is designed to estimate the degree to which diet induces or suppresses inflammatory pathways, by leveraging dietary intake data, which attempts to provide an accurate picture of foods regularly consumed by an individual. Hypothetically, the ideal strategy for assessing an individual’s dietary inflammatory potential would be to determine the amount of energy and nutrients available for metabolism from foods consumed over a long period of time.23 In general, this is not a feasible data collection approach, especially at the scale required by large population-based studies. Thus, the ability to estimate the inflammatory potential of diet with widely used dietary intake tools provides an appealing alternative.22
As described in detail by Shivappa et al. (2014), the DII is based on a meta-analysis of 1,943 articles that assessed the impact of whole foods, nutrients, and bioactive compounds on inflammatory markers, specifically interleukin(IL)-1β, IL-4, IL-6, IL-10, tumor necrosis factor α, and C-reactive protein (CRP). Eligible articles were weighted based on study design, and food parameters were assigned an inflammatory effect score based on whether associations with biomarkers were pro- or anti-inflammatory. A negative inflammatory effect score means that a food parameter is considered anti-inflammatory (e.g. fiber, polyunsaturated fatty acids), and a positive inflammatory effect score means that a food parameter is considered pro-inflammatory (e.g. saturated fat, total kilocalories). Likewise, an individual’s total DII score can range from negative values, indicating an overall anti-inflammatory diet, to positive values, indicating an overall pro-inflammatory diet. The association between DII score and circulating levels of inflammatory markers, CRP and IL-6, has been validated in previous population-based studies, including the National Health and Nutrition Examination Survey (NHANES).24–27
The use of the DII differs from nutritional epidemiology studies that assess single nutrients in isolation, which are unable to consider complex interactions between foods that may impact inflammation differently.28 The DII approach also differs from methods examining dietary patterns (e.g. Mediterranean diet, Western diet), which group individuals based on consumption of certain foods in combination but are not characterized by their potential to influence underlying biological mechanisms.29 DII score has previously been associated with circulating levels of pro-inflammatory biomarkers,24,30 depression and other measures of mental health,31–35 cardiovascular disease and metabolic conditions,36–38 and multiple cancers.39–41
This study examines whether food security status is associated with DII score within a large representative sample of lower-income US adults. This study also investigates whether this association is moderated by important demographic characteristics: marital status, sex, and age group. Evidence indicates that the experience of food insecurity differs by demographic factors. For example, a socioeconomically disadvantaged single-mother that is food insecure may be likely to decrease her consumption of food or go without in order to provide for her children42 and, therefore, may be more likely to consume calorically-dense, nutrient poor foods with a higher inflammatory potential in order to feel satiated. Alternatively, a higher prevalence of functional impairment among food insecure elderly persons contributes to poorer food utilization (e.g. poorer nutritional status and difficulty obtaining food assistance) compared to other age groups.43 Thus, it is reasonable to hypothesize that disparate access to various resources across demographic groups may moderate the association of food security status with dietary inflammatory potential.
Materials and Methods
Data
Data for this study came from the National Health and Nutrition Examination Survey (NHANES) 2007 to 2014 cross-sectional samples, collected in two-year cycles. These were the most recent cycles that had released the variables of interest included in this study. Approval for NHANES and data collection was provided by the National Center for Health Statistics Research Ethics Review Board. Secondary analysis of NHANES data was determined to be exempt from institutional review board by the University of Wisconsin-Madison Health Sciences Institutional Review Board. NHANES included two components: an in-home interview and a health examination. Signed consent for both was obtained from all participants during the in-home interview.44 A complex, multistage probability sampling design was used to select samples representative of the US population. Analyses for this study were limited to adults aged 20 years and older, since this is the age restriction set by NHANES for its study participants to receive questionnaires specific to adults. The sample was also limited to those with an income-to-poverty ratio ≤3.00. The income-to-poverty ratio reflects the ratio of an individual’s household income to the federal poverty level; this level is set by the federal government each year and is considered the minimum household income required to cover basic living expenses in the US, adjusted for household size. Those that have an income-to-poverty level <1.00 are considered to live in poverty.45 Finally, the sample was restricted to those with non-missing information for variables of interest.
Measures
Dietary Inflammatory Index (DII) Score –DII score was calculated using previously published methods and reference values for global mean consumption and standard deviations described by Shivappa et al.22 In brief, DII score was determined using an average of the NHANES dietary intake data from two 24-hour dietary recalls. The first dietary recall was collected in person during the medical examination and the second was collected via telephone 3 to 10 days later. These data were used to calculate average individual consumption values for the following DII food parameters: total energy (kcal); carbohydrates (g); fat (g); protein (g); alcohol (g); caffeine (mg); fiber (g); cholesterol (mg); total saturated, monounsaturated, and polyunsaturated fatty acids (g); n-3 and n-6 polyunsaturated fatty acids (g); niacin (mg); riboflavin (mg); thiamin (mg); vitamins A (μg), B6 (mg), B12 (μg), C (mg), D (μg), E (mg); β-carotene (μg); iron (mg); magnesium (mg); selenium (μg); zinc (mg); and folate (μg).
Each individual consumption value was then subtracted from previously published global mean daily consumption estimates and this difference was divided by the global standard deviation for each food parameter to create a standardized Z-score. Next, these Z-scores were converted to percentiles and centered by doubling their value then subtracting 1 in order to minimize the effect of right skewing. The centered-percentile of each food parameter was then multiplied by the respective inflammatory effect score, also reported by Shivappa et al.22 Finally, all of the individual food parameter DII scores were summed to create the ‘overall DII score’ for an individual. In addition to the continuous DII score, individuals were assigned to DII quintiles. Weighted quintiles of the overall DII score were calculated using the entire NHANES adult cohort distribution with non-missing data for each of the food parameters included in dietary recalls.
Food Security Status
In NHANES, food security status was measured at the household level using the US Food Security Survey Module.46 This module is a questionnaire that assesses level of food security (high, marginal, low, or very low) using a series of questions regarding the influence of financial hardship on quantity and quality of food consumed, as well as mental distress due to household food supply. A 10-item questionnaire was used for households without children less than 18 years of age. For households with children, an 18-item questionnaire that incorporates additional questions specific to child food security was used. Affirmative answers to questionnaire items were summed to categorize households, where a score of 0 indicates high food security, a score of 1–2 indicates marginal food security, a score of 3–5 for households without children or a score of 3–7 for households with children indicates low food security, and a score of 6–10 for households without children or a score of 8–18 for households with children indicates very low food security.
When evaluating interactions between food security status and demographic factors, a binary measure of food security status (secure vs. insecure) was used to facilitate interpretation. Those living in households with marginal to very low food security were considered food insecure. Those living in households with high food security were considered food secure. This binary measure of food security status is consistent with previous literature that indicates even marginal food security is associated with worse health outcomes than high food security.47
Covariates
Due to the potential for confounding, a number of covariates were considered. Demographic covariates included age group (20 to <40, 40 to <65, 65 and older), race/ethnicity (non-Hispanic White, non-Hispanic Black, Hispanic, other or multi-racial), sex (male, female), educational status (<high school, high school graduate or equivalent, some college, college graduate and above), and marital status (married or living with partner; never married; widowed, separated, or divorced). Socioeconomic covariates included employment status (working, not working and not looking for work, not working and looking for work), health insurance status (private, subsidized, uninsured), and income-to-poverty ratio. In addition, using a conservative approach, perceived health status (excellent or very good; good; poor or fair) was also considered due to its potential for confounding. Self-report health status is consistently associated with mortality48 and may be an indicator of health behaviors including dietary consumption. Furthermore, chronic conditions that contribute to poorer health status can be economically burdensome and are hypothesized to have a bidirectional association with food insecurity.49
Statistical Analysis
All analyses were conducted using SAS® software, version 9.4.50 SAS® software survey design procedures accounted for the effects of stratification and clustering used in the NHANES study design. Six-year sampling weights were calculated by multiplying the sampling weights provided by NHANES for two-year cycles by one-third. Applying sampling weights accounted for (a) unequal probabilities of selection across NHANES participants, (b) non-response specific to dietary recalls 1 and 2, and (c) the proportion of weekend-weekday combinations of dietary recalls 1 and 2 across individuals since proportionally more exams occur on weekend days than on weekdays.
Type III F-tests or χ2 tests compared sample characteristics by food security status. Univariate linear regression was used to determine the distribution of consumption for each food parameter included in the DII (mean, 95% confidence intervals) by food security status. Next, a series of multivariate linear regression models were used to determine the association between food security status and DII score. Model 1 accounts for demographic characteristics; Model 2 accounts for all covariates in Model 1 plus a number of socioeconomic factors, and; Model 3 accounts for self-reported health status in addition to all covariates considered in Model 2. In addition, the potential of demographic characteristics to moderate the association between food security status and DII score was examined. To do so, interaction terms between demographic characteristics (marital status, sex, and age group) and the binary measure of food security status were tested separately by adding them individually to Model 3.
Although total energy intake is endogenous to the DII, it is often used as a covariate in diet studies to account for nutrient density.51 Therefore, the average of total kilocalories consumed (determined using two 24-hour dietary recalls) was included so that total energy intake did not confound associations between food security status and DII score.
Results
Among all NHANES 2007–2014 participants, 11,072 were ≥20 years of age and had an income-to-poverty ratio ≤3.00. Of these participants 10,630 had non-missing data for variables of interest. Table 1 presents descriptive statistics by food security status. Those who fared worse socioeconomically tended to be less food secure. The percent of individuals not working but looking for work was three times as high among those with very low food security than among those with high food security (7.8% vs. 2.6%). The percent of uninsured individuals among those with very low food security was also higher than among those with high food security (40.4% vs. 24.0%). Food security status did not vary by sex, but White non-Hispanics, those ≥65 years of age, and married individuals were less likely to experience food insecurity. In addition, comparing those with low food security to those with very low food security, there were fewer but some noticeable differences. For example, among those with low food security, 52.0% were married or living with a partner compared to 43.9% of individuals with very low food security. Also, 35.9% of those with low food security reported fair or poor health compared to 40.9% of individuals with very low food security.
Table 1.
Descriptive characteristics NHANESa 2007 to 2014 adults aged ≥20 years with an income-to-poverty ratio ≤3.00b by food security status
| Characteristics | Food Security Status |
P-valuec | |||
|---|---|---|---|---|---|
| High Food Security | Marginal Food Security | Low Food Security | Very Low Food Security | ||
| n= 6,117 | n= 1,531 | n= 1,842 | n= 1,140 | ||
| Age in years (%) | <0.0001 | ||||
| 20 to <40 | 38.8 | 47.2 | 52.3 | 48.0 | |
| 40 to <65 | 35.8 | 40.8 | 39.8 | 43.5 | |
| 65 and older | 25.4 | 12.0 | 7.9 | 8.5 | |
| Male (%) | 45.2 | 44.3 | 45.8 | 44.7 | 0.9519 |
| Race/ethnicity (%) | <0.0001 | ||||
| Non-Hispanic White | 66.9 | 44.4 | 44.0 | 50.1 | |
| Hispanic | 14.7 | 27.4 | 32.9 | 23.7 | |
| Non-Hispanic Black | 12.1 | 19.5 | 18.0 | 20.4 | |
| Other or multi-race/ethnicity | 6.4 | 8.8 | 5.2 | 5.7 | |
| Education (%) | <0.0001 | ||||
| College grad and above | 18.2 | 12.2 | 5.9 | 5.3 | |
| Associates degree or some college | 31.8 | 32.9 | 28.5 | 33.4 | |
| High school or equivalent | 28.0 | 28.4 | 27.3 | 28.6 | |
| <High school | 22.0 | 26.5 | 38.3 | 32.7 | |
| Marital status (%) | 0.0012 | ||||
| Married or living with partner | 55.4 | 50.6 | 52.0 | 43.9 | |
| Never married | 21.9 | 25.3 | 26.1 | 27.5 | |
| Widowed, divorced, or separated | 22.8 | 24.1 | 22.0 | 28.6 | |
| Income-to-poverty ratiod (%) | <0.0001 | ||||
| ≤1.00 | 21.2 | 37.7 | 47.7 | 52.5 | |
| >1.00 to ≤2.00 | 41.7 | 42.6 | 39.4 | 37.4 | |
| >2.00 to ≤3.00 | 37.1 | 19.7 | 12.9 | 10.0 | |
| Employment status (%) | <0.0001 | ||||
| Employed | 51.2 | 54.3 | 49.9 | 45.0 | |
| Not working and not looking for work | 46.1 | 40.4 | 44.6 | 47.2 | |
| Not working and looking for work | 2.6 | 5.3 | 5.5 | 7.8 | |
| Health insurance status (%) | <0.0001 | ||||
| Private | 51.9 | 33.7 | 25.9 | 20.9 | |
| Subsidized | 24.1 | 28.9 | 34.2 | 38.7 | |
| Uninsured | 24.0 | 37.5 | 39.9 | 40.4 | |
| Perceived health status (%) | <0.0001 | ||||
| Excellent or very good | 43.0 | 34.2 | 27.3 | 24.3 | |
| Good | 36.8 | 39.7 | 36.8 | 34.8 | |
| Fair or poor | 20.2 | 26.1 | 35.9 | 40.9 | |
| DIIe score, mean (95% CI) | −0.28 (−0.38, −0.19) | −0.06 (−0.23, 0.12) | 0.08 (−0.07, 0.23) | 0.28 (0.10, 0.46) | <0.0001 |
| DII quintilef (%) | <0.0001 | ||||
| Q1 | 20.2 | 19.2 | 14.7 | 12.5 | |
| Q2 | 20.6 | 17.3 | 20.0 | 17.8 | |
| Q3 | 20.8 | 19.4 | 19.2 | 18.3 | |
| Q4 | 18.8 | 20.9 | 21.9 | 22.4 | |
| Q5 | 19.6 | 23.2 | 24.2 | 29.0 | |
National Health and Nutrition Examination Survey
Table percents are weighted
Rao-Scott χ² and F-test
Reflects the ratio of an individual’s household income to the federal poverty level; this level is set by the federal government each year and is considered the minimum household income required to cover basic living expenses in the US, adjusted for household size. Those that have an income-to-poverty level <1.00 are considered to live in poverty (Levy et al. 2008).
Dietary Inflammatory Index
DII quintile determined by weighted distribution of NHANES population DII scores with non-missing dietary data;
Table 2 provides the distribution of DII food parameters by food security status. Differential consumption of multiple DII food parameters across food security status groups may contribute to food secure individuals having a lower DII score than food insecure individuals. Highly food secure individuals reported a slightly higher intake of fiber, an anti-inflammatory food parameter, and lower consumption of carbohydrates, a pro-inflammatory food parameter, compared to all three subsequent levels of food security. Those with low or very low food security had a lower intake of several anti-inflammatory food parameters compared to those with greater food security. These include fiber, Vitamin A, B6, C and E, β-carotene, and magnesium.
Table 2.
Mean and 95% confidence interval for food parameter DIIa by food security status among adults ≥20 years with an income-to-poverty ratio ≤3.00, NHANESb 2007–2014
| Food Parameters | DII Inflammatory Effect Scorec | High Food Security | Marginal Food Security | Low Food Security | Very Low Food Security | P-valued | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| n= 6,117 | n= 1,531 | n= 1,842 | n= 1,140 | |||||||
| Mean | 95% CI | Mean | 95% CI | Mean | 95% CI | Mean | 95% CI | |||
| Energy (total kcal) | 0.180 | 2,026 | 1,985 to 2,066 | 2,053 | 1,984 to 2,122 | 2,078 | 2,006 to 2,149 | 2,104 | 2,006 to 2,203 | 0.27 |
| Carbohydrates (g)** | 0.097 | 249 | 244 to 254 | 254 | 245 to 263 | 262 | 253 to 272 | 265 | 253 to 278 | 0.0069 |
| Fat (g) | 0.298 | 76.0 | 74.3 to 77.7 | 75.6 | 72.5 to 78.7 | 76.2 | 73.1 to 79.4 | 77.5 | 73.5 to 81.6 | 0.86 |
| Protein (g) | 0.021 | 79.5 | 77.9 to 81.2 | 79.9 | 76.8 to 83.0 | 79.6 | 76.9 to 82.3 | 77.4 | 73.7 to 81.1 | 0.66 |
| Alcohol (g) | −0.278 | 7.6 | 6.6 to 8.7 | 8.7 | 6.1 to 11.3 | 6.6 | 4.9 to 8.3 | 8.2 | 5.5 to 11.0 | 0.32 |
| Caffeine (mg)* | −0.110 | 0.15 | 0.14 to 0.16 | 0.14 | 0.12 to 0.15 | 0.14 | 0.12 to 0.15 | 0.17 | 0.15 to 0.15 | 0.0128 |
| Fiber (g)** | −0.663 | 16.4 | 15.9 to 16.8 | 15.9 | 15.1 to 16.6 | 15.8 | 15.0 to 16.5 | 15.0 | 14.1 to 16.0 | 0.0071 |
| Cholesterol (mg) | 0.110 | 278 | 271 to 286 | 281 | 264 to 299 | 293 | 276 to 310 | 277 | 259 to 296 | 0.31 |
| Saturated fat (g) | 0.373 | 24.8 | 24.2 to 25.4 | 24.9 | 23.8 to 26.1 | 25.2 | 24.1 to 26.3 | 25.8 | 24.5 to 27.1 | 0.49 |
| MUFAe (total g) | −0.009 | 27.2 | 26.6 to 27.8 | 27.0 | 25.9 to 25.8 | 27.1 | 26.0 to 25.9 | 27.3 | 25.8 to 26.4 | 0.99 |
| PUFAf (total g) | −0.337 | 17.2 | 16.8 to 17.6 | 16.7 | 15.9 to 17.4 | 17.0 | 16.2 to 17.7 | 17.4 | 16.2 to 18.6 | 0.49 |
| n-3 PUFA (g) | −0.436 | 0.13 | 0.12 to 0.14 | 0.14 | 0.11 to 0.17 | 0.13 | 0.11 to 0.15 | 0.12 | 0.10 to 0.15 | 0.89 |
| n-6 PUFA (g) | −0.159 | 15.2 | 14.8 to 15.5 | 14.7 | 14.0 to 15.4 | 15.0 | 14.3 to 15.6 | 15.4 | 14.3 to 16.5 | 0.47 |
| Niacin (mg) | −0.246 | 25.0 | 24.4 to 25.5 | 25.1 | 24.1 to 26.1 | 24.5 | 23.7 to 25.4 | 25.1 | 23.7 to 26.4 | 0.63 |
| Riboflavin (mg)** | −0.068 | 2.10 | 2.05 to 2.15 | 2.00 | 1.89 to 2.11 | 1.97 | 1.88 to 2.05 | 2.05 | 1.92 to 2.18 | 0.0099 |
| Thiamin (mg) | −0.098 | 1.62 | 1.57 to 1.66 | 1.56 | 1.49 to 1.63 | 1.56 | 1.49 to 1.63 | 1.59 | 1.49 to 1.68 | 0.29 |
| Vitamin A, RAEg (μg)*** | −0.401 | 633 | 609 to 656 | 582 | 536 to 629 | 546 | 508 to 583 | 543 | 510 to 577 | <0.0001 |
| Vitamin B6 (mg)* μg) | −0.365 | 2.08 | 2.02 to 2.14 | 2.06 | 1.95 to 2.17 | 1.96 | 1.89 to 2.03 | 1.98 | 1.86 to 2.10 | 0.0109 |
| Vitamin B12 (μg)* | 0.106 | 6.29 | 6.08 to 6.51 | 5.96 | 5.54 to 6.38 | 5.70 | 5.20 to 6.20 | 6.49 | 5.77 to 7.20 | 0.0399 |
| Vitamin C (mg)** | −0.424 | 83.2 | 79.4 to 87.1 | 81.2 | 74.2 to 88.2 | 82.1 | −2.1 to 87.7 | 69.6 | −12.4 to 76.9 | 0.0026 |
| Vitamin D (μg) | −0.446 | 4.67 | 4.49 to 4.85 | 4.47 | 3.99 to 4.95 | 4.44 | 4.13 to 4.75 | 4.59 | 4.20 to 4.98 | 0.49 |
| Vitamin E (mg)*** | −0.419 | 8.52 | 8.22 to 8.83 | 8.01 | 7.36 to 8.66 | 7.52 | 7.08 to 7.97 | 7.63 | 7.07 to 8.20 | <0.0001 |
| β-Carotene (μg)*** | −0.584 | 2,135 | 2,003 to 2,268 | 1,945 | 1,703 to 2,188 | 1,524 | 1,348 to 1,700 | 1,542 | 1,332 to 1,753 | <0.0001 |
| Iron (mg) | 0.032 | 14.9 | 14.6 to 15.3 | 14.8 | 13.9 to 15.7 | 14.6 | 14.0 to 15.2 | 14.6 | 13.9 to 15.4 | 0.67 |
| Magnesium (mg)** | −0.484 | 287 | 281 to 294 | 277 | 265 to 288 | 273 | 263 to 284 | 268 | 253 to 282 | 0.0078 |
| Selenium (μg) | −0.191 | 110 | 108 to 113 | 110 | 106 to 114 | 111 | 107 to 115 | 108 | 102 to 114 | 0.84 |
| Zing (mg) | −0.313 | 11.3 | 11.0 to 11.6 | 11.0 | 10.5 to 11.5 | 11.1 | 10.7 to 11.6 | 10.8 | 10.2 to 11.4 | 0.29 |
| Folate (μg) | −0.190 | 402 | 390 to 414 | 388 | 366 to 410 | 384 | 367 to 402 | 386 | 364 to 407 | 0.15 |
Dietary Inflammatory Index
National Health and Nutrition Examination Survey
Values range from negative, indicating an anti-inflammatory effect, to positive, indicating a pro-inflammatory effect; source: Shivappa et al. (2014)
F-test
Monounsaturatted fatty acids
Polyunsatured fatty acids
Retinol Activity Equivalents
for P <0.05;
for P <0.01;
for P <0.001
Table 3 presents results for regression of DII score on food security status. Even in fully adjusted models, food security status was associated with DII score (p-value = 0.0033). Individuals with very low food security had a 0.31 (95% CI=0.12 to 0.49) higher DII score than those with high food security. Across the three models tested, the association between food security status and DII did not vary significantly following multivariate adjustment. Other factors were also observed to be associated with DII score in the fully adjusted model. Older individuals, males, and those with higher education, private health insurance and better health status had lower DII scores than other groups. In addition, compared to non-Hispanic Whites, non-Hispanic Black individuals had a 0.19 (0.04, 0.34) higher DII score, whereas Hispanic individuals had a 0.55 (0.34, 0.76) lower DII score (p-value < 0.0001). Results in Table 3 remain unchanged when accounting for total energy intake (data not shown).
Table 3.
Difference in DIIa score by food security status and other characteristics among adults ≥20 years with an income-to-poverty ratio <3.00, NHANESb 2007 to 2014
| Characteristics | Model 1c | Model 2c | Model 3c | ||||||
|---|---|---|---|---|---|---|---|---|---|
| β | 95% CI | P-valued | β | 95% CI | P-valued | β | 95% CI | P-valued | |
| Food Security Status | <.0001 | 0.0011 | 0.0033 | ||||||
| High Food Security | Ref. | Ref. | Ref. | ||||||
| Marginal Food Security | 0.20*** | 0.02, 0.37 | 0.15*** | −0.02, 0.33 | 0.14*** | −0.04, 0.32 | |||
| Low Food Security | 0.27*** | 0.12, 0.41 | 0.20*** | 0.05, 0.34 | 0.17*** | 0.02, 0.32 | |||
| Very Low Food Security | 0.43*** | 0.25, 0.62 | 0.34*** | 0.15, 0.53 | 0.31*** | 0.12, 0.49 | |||
| Age | 0.2015 | 0.1356 | 0.0492 | ||||||
| 20 to <40 | Ref. | Ref. | Ref. | ||||||
| 40 to <65 | −0.11 | −0.23, 0.02 | −0.12 | −0.25, 0.02 | −0.16* | −0.29, −0.03 | |||
| 65 and older | −0.12 | −0.29, 0.05 | −0.19 | −0.39, 0.01 | −0.21* | −0.41, −0.02 | |||
| Sex | <.0001 | <0.0001 | <0.0001 | ||||||
| Female | Ref. | Ref. | Ref. | ||||||
| Male | −0.92*** | −1.03, −0.82 | −0.90*** | −1.01, −0.80 | −0.90*** | −1.00, −0.79 | |||
| Race/ethnicity | <.0001 | <0.0001 | <0.0001 | ||||||
| Non-Hispanic White | Ref. | Ref. | Ref. | ||||||
| Hispanic | −0.50*** | −0.70, −0.30 | −0.51*** | −0.72, −0.31 | −0.55*** | −0.76, −0.34 | |||
| Non-Hispanic Black | 0.22*** | 0.06, 0.37 | 0.20*** | 0.04, 0.35 | 0.19*** | 0.04, 0.34 | |||
| Other or Multi-race/ethnicity | −0.18*** | −0.39, 0.03 | −0.20*** | −0.41, 0.01 | −0.21*** | −0.42, 0.00 | |||
| Education | <.0001 | <0.0001 | <0.0001 | ||||||
| College grad and above | Ref. | Ref. | Ref. | ||||||
| Associates degree or some college | 0.45*** | 0.26, 0.63 | 0.42*** | 0.24, 0.60 | 0.38*** | 0.20, 0.56 | |||
| High school or equivalent | 0.93*** | 0.74, 1.13 | 0.88*** | 0.69, 1.08 | 0.82*** | 0.62, 1.02 | |||
| <High school | 1.12*** | 0.95, 1.30 | 1.03*** | 0.86, 1.20 | 0.95*** | 0.77, 1.14 | |||
| Marital status | 0.2838 | 0.5425 | 0.6033 | ||||||
| Married or living with partner | Ref. | Ref. | Ref. | ||||||
| Never married | 0.03 | −0.13, 0.18 | −0.01 | −0.17, 0.14 | 0.00 | −0.16, 0.15 | |||
| Widowed, divorced, or separated | 0.10 | −0.03, 0.23 | 0.07 | −0.07, 0.21 | 0.07 | −0.07, 0.20 | |||
| Income-to-Poverty Ratio | −0.05 | −0.14, 0.04 | 0.2304 | −0.05 | −0.14, 0.04 | 0.3059 | |||
| Employment status | 0.1372 | 0.2906 | |||||||
| Employed | Ref. | Ref. | |||||||
| Not working and not looking for work | 0.13 | −0.01, 0.27 | 0.10 | −0.04, 0.24 | |||||
| Not working and looking for work | 0.07 | −0.20, 0.34 | 0.08 | −0.19, 0.35 | |||||
| Health insurance status | 0.0015 | 0.0040 | |||||||
| Private | Ref. | Ref. | |||||||
| Subsidized | 0.23*** | 0.11, 0.36 | 0.21*** | 0.08, 0.34 | |||||
| Uninsured | 0.16*** | 0.01, 0.30 | 0.15*** | 0.01, 0.29 | |||||
| Perceived health status | 0.0021 | ||||||||
| Excellent or very good | Ref. | ||||||||
| Good | 0.23*** | 0.10, 0.37 | |||||||
| Fair or poor | 0.31*** | 0.12, 0.49 | |||||||
Dietary Inflammatory Index
National Health and Nutrition Examination Survey
Model 1 adjusts for demographic characteristics; Model 2 adjusts for all covariates in Model 1 plus socioeconomic factors; Model 3 adjusts for all covariates in Model 2 plus health status
F-test in a joint model
for p-values < 0.05
for p-values < 0.005
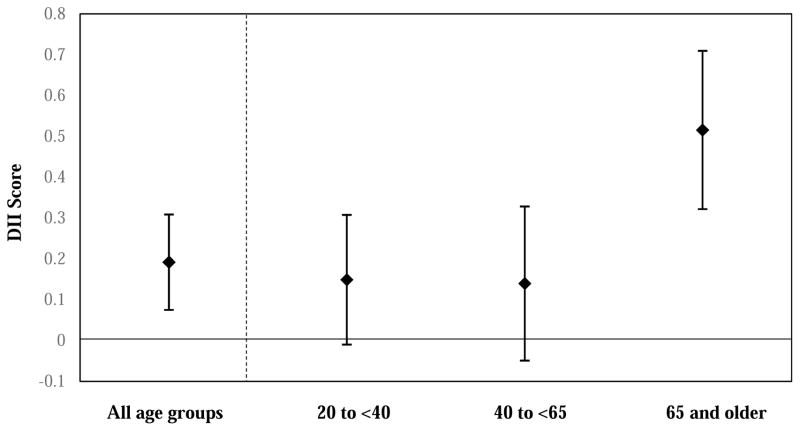
When testing whether demographic factors moderate the association between the binary measure of food security status and DII score, interactions with marital status (interaction term p-value = 0.16) and sex (interaction term p-value = 0.46) were not significant. However, as presented in Figure 1, age group moderated the association between food security status and DII score (interaction term p-value = 0.0103). The association between food insecurity and DII score was 0.15 (−0.01 to 0.31) for those aged 20 to <45 years, 0.14 (−0.05 to 0.33) for those aged 45 to <65 years, and 0.52 (0.32 to 0.71) for those aged 65 years and older. Results of moderation by demographic factors remain unchanged when accounting for total energy intake (data not shown).
Figure 1.
The association between food insecuritya and DIIb score among lower-incomec adults and moderation by age groupd, e
aThose that reported marginal to very low food security were considered food insecure. Those that reported high food security were considered food secure.
bDietary Inflammatory Index
cAdults with an income-to-poverty ratio ≤3.00
dAdjusted for sex, race/ethnicity (non-Hispanic White, non-Hispanic Black, Hispanic, other), education (<high school, high school degree or equivalent, Associates degree or some college, collage and above), marital status (married or living with partner; separated, widowed, or divorced; never married), income-to-poverty ratio, employment status (employed, not working and not looking for work, not working and looking for work), health insurance status (private, subsidized, uninsured), and perceived health status (excellent or very good, good, fair or poor).
eThe p-value for the interaction between food insecurity and age group was 0.0103.
Discussion
While food insecurity has been associated with a number of adverse health outcomes, including poor mental health,52 there is a need for greater evidence regarding underlying biological mechanisms in order to ascertain the causal nature of these associations. One hypothesis is that food insecurity may influence inflammatory pathways associated with chronic disease due to psychosocial strain or diet quality.53 This study is among the first to examine whether food insecurity is associated with dietary inflammatory potential, measured using the Dietary Inflammatory Index (DII). The DII is a composite dietary index that includes components associated with inflammation, and is related to a number of negative health outcomes.31–41
Among a representative sample of lower-income US adults, those with low or very low food security had a higher DII score than those with high food security, even after accounting for demographic and socioeconomic factors, as well as perceived health status. This indicates that food security status may be associated with dietary inflammatory potential. Interestingly, results also suggest that age moderates this association. While food insecurity was associated with a higher DII score for all age groups, DII score was much higher for food insecure individuals aged 65 years and older compared to younger age groups. When looking at the distribution of DII food parameters across food security status, it appears that a lower consumption of fiber, Vitamin A, B6, C and E, β-carotene, and magnesium, and a higher consumption of carbohydrates among those that have low or very low food security may contribute to the observed associations. This is consistent with previous findings regarding diet quality among food insecure individuals.20
To contextualize the findings of this study, the overall magnitude of the association between food security status and DII score in this study is similar to that detected for occupational status in previous work. Wirth and colleagues,54 for example, observed that day workers had a lower mean DII score (0.86; 95% CI: 0.79 – 0.94) than those with any evening/night shiftwork (1.01; 95% CI: 0.89 to 1.13) or rotating shifts (1.07; 95% CI: 0.92 – 1.22).
One other known study has evaluated whether indicators of systemic inflammation may be associated with food security status. Gowda and colleagues observed that food insecurity was associated with lower levels of serum folate, considered anti-inflammatory by the DII.53 However, they did not find food insecurity to be associated with vitamin A or B12 levels, considered anti- and pro-inflammatory by the DII, respectively. The previous study differs from this current investigation; it took a single nutrient approach examining the association between food security status and serum levels of specific micronutrients, rather than the DII as a whole. Another important distinction is that this analysis was restricted to lower-income individuals, whereas Gowda et al. (2012) did not use an income cutoff. If those with an income-to-poverty ratio >3.00 had not been excluded in this analysis, there would likely be an even larger difference in DII score when comparing highly food secure individuals to those with lower food security. This is because higher-income adults tend to consume diets of better quality than lower-income adults, as demonstrated in a previous study using NHANES data.55
Of note, results of this study also indicate that food insecurity may lead to a particularly high DII score among those aged 65 years and older. Traits that are more common among elderly persons may contribute to food insecurity having a greater influence on diet. A higher prevalence of functional impairment due to disability, cognitive decline, and aging is of particular concern. The association between frailty and malnutrition is well established.56 Functional impairment contributes to elderly persons having poorer food utilization, including poorer nutritional status and greater difficulty obtaining food assistance, than younger age groups.43 Additionally, a lack of social resources, due to social isolation or less social support or capital, may play a role. Among older persons, having fewer social resources is associated with decreased transportation, weaker community ties, an inability to prepare meals, and increased nutritional risk.57 It is hypothesized that these factors may limit one’s access to healthy foods, decrease healthy eating practices, and increase consumption of convenient, yet nutrient poor foods that lead to a diet with a higher pro-inflammatory potential. Further research examining these mechanisms is needed.
Strengths of this study include the use of a large dataset that accommodated testing moderation of the association between food security status and DII score by demographic factors. Furthermore, these data are representative of non-institutionalized lower-income US adults and may be useful in comparisons to low income populations in other Western countries with similar dietary behaviors. The use of the DII to estimate the association between food insecurity and dietary inflammatory potential may be considered both a strength and a weakness. The DII’s ability to estimate total dietary inflammatory potential goes beyond a single nutrient approach to investigate whether an individual’s aggregate diet may contribute to mechanisms of chronic inflammation and subsequent associations with adverse health. However, relying on 24-hour dietary recalls to determine habitual dietary intake is a weakness. Even though this study assessed dietary intake using an average of two 24-hour recalls on different days of the week, this may not capture the temporal variability of dietary consumption. It is well established that 24-hour dietary recalls are an imperfect measure when assessing diet; they are prone to differential underreporting across population subgroups,58 and they are a less precise tool than daily diary records.59 While the DII developed by Shivappa et al. 2014, includes 45 food parameters, only 28 food parameters are available from the NHANES 24-hour dietary recalls and included in the calculation of DII score for this study, which is a limitation. However, the missing food parameters likely make up a small proportion of total nutrients consumed within the sample population (e.g. turmeric, saffron, green tea), and previous studies have validated the association between the DII and circulating levels of inflammatory markers even when limited by the number of food parameters available,25 as in this study.
Limitations of this study also include the cross-sectional nature of NHANES data, which precludes causal interpretation of results. In addition, social desirability bias may lead to underreporting of food insecurity and subsequently result in underestimating the association between food security status and dietary inflammatory potential. Finally, random measurement error has the potential to decrease precision and bias results towards the null.60 The inability to account for potential unobserved confounders may upwardly bias results.
Conclusion
Results of this study suggest that food insecurity may be associated with dietary inflammatory potential, which has been linked with a number of chronic diseases. Findings further suggest that elderly persons may be especially at risk for a high dietary inflammatory potential when struggling with food insecurity. More evidence is needed to determine whether reducing dietary inflammatory potential among food insecure populations is a viable strategy for addressing health disparities. While previous studies have validated the association between the DII and circulating levels of inflammatory markers,24–27 these studies include only two markers of inflammation, CRP and IL-6, and are limited to relatively homogenous populations. Moving forward, studies may consider validation of the DII using additional inflammatory markers, and examine whether the DII is associated with circulating levels of the specific micronutrients it is designed to capture. Given the differential associations observed by age, future research may consider whether DII score among food insecure older adults is a function of worse scores across all DII components, or if older adults have worse scores for specific components compared to younger individuals. To effectively address health disparities, it is important to understand the underlying biological mechanisms by which psychosocial stressors like food insecurity influence adverse health outcomes. Empirically-based strategies are necessary to ameliorate the complex chronic conditions associated with food insecurity.
Research Snapshot.
Research Question
Is food security status associated with dietary inflammatory potential?
Key Findings
In this cross-sectional cohort of 10,630 adults from the United States National Health and Nutrition Examination Survey (NHANES) 2007–2014 cycles, food security status was associated with dietary inflammatory potential, measured using the Dietary Inflammatory Index (DII). When accounting for socioeconomic status, demographic factors, and health status, DII score was higher at greater levels of food insecurity (p-value = 0.0033). Those with very low food security had a 0.31 (95% CI=0.12 to 0.49) higher DII score than those with high food security.
Footnotes
Author Contributions: RSB designed this study with contribution from KMM. RSB analyzed the data with contribution from KMM and MP. RSB interpreted results with contribution from KMM, MP, SAR, LMB, and DBE. RSB wrote the first draft with contribution from KMM. All authors contributed to critical revision of subsequent drafts and approved of the final version.
Conflict of Interest Disclosures:
Authors declare no conflicts of interest.
Financial & Funding Disclosures:
Funding that supported RSB’s work on this research comes from the National Institute of Health (NIH) Eunice Kennedy Shriver National Institute for Child Health and Development (NICHD) Training Grant (Demography and Ecology): T32 HD007014. KMM’s work on this research was supported by the Center for Demography and Ecology at the University of Wisconsin-Madison (P2C HD047873), the Collaborative Center for Health Equity Administration (WPP3086), the National Institutes of Health, the National Center for Advancing Translational Science CTSA award UL1TR000427, and the National Institute for Minority Health and Health Disparities award 1P60MD0003428. None of these funding sources had any role in study design; collection, analysis, or interpretation of data; writing the report; or the decision to submit the report for publication. MP, SR, LB, and DBE have no funding sources to report that supported work on this manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.National Research Council; Committee on National Statistics, Division of Behavioral and Social Sciences and Education, editor. Food Insecurity and Hunger in the United States: An Assessment of the Measure. Washington (DC): National Academies Press (US); 2006. [Google Scholar]
- 2.Coleman-Jensen A, Rabbitt MP, Gregory C, Singh A. Household Food Security in the United States in 2014. U.S. Department of Agriculture, Economic Research Service; Washington, DC: 2015. [Google Scholar]
- 3.Stuff JE, Casey PH, Connell CL, et al. Household Food Insecurity and Obesity, Chronic Disease, and Chronic Disease Risk Factors. J Hunger Environ Nutr. 2006;1(2):43–62. [Google Scholar]
- 4.Pan L, Sherry B, Njai R, Blanck HM. Food Insecurity Is Associated with Obesity among US Adults in 12 States. J Acad Nutr Diet. 2012;112(9):1403–1409. doi: 10.1016/j.jand.2012.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gundersen C, Ziliak JP. Food Insecurity and Health Outcomes. Health Aff (Millwood) 2015;34(11):1830–1839. doi: 10.1377/hlthaff.2015.0645. [DOI] [PubMed] [Google Scholar]
- 6.Tabas I, Glass CK. Anti-Inflammatory Therapy in Chronic Disease: Challenges and Opportunities. Science. 2013;339(6116):166–172. doi: 10.1126/science.1230720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420(6917):860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mason JC, Libby P. Cardiovascular disease in patients with chronic inflammation: mechanisms underlying premature cardiovascular events in rheumatologic conditions. Eur Heart J. 2014 Nov;36(8):482–489. doi: 10.1093/eurheartj/ehu403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Donath MY. Targeting inflammation in the treatment of type 2 diabetes: time to start. Nat Rev Drug Discov. 2014;13(6):465–476. doi: 10.1038/nrd4275. [DOI] [PubMed] [Google Scholar]
- 10.Berg AH, Scherer PE. Adipose Tissue, Inflammation, and Cardiovascular Disease. Circ Res. 2005;96(9):939–949. doi: 10.1161/01.RES.0000163635.62927.34. [DOI] [PubMed] [Google Scholar]
- 11.Khansari PS, Sperlagh B. Inflammation in neurological and psychiatric diseases. Inflammopharmacology. 2012;20(3):103–107. doi: 10.1007/s10787-012-0124-x. [DOI] [PubMed] [Google Scholar]
- 12.Maes M, Song C, Lin A, et al. The effects of psychological stress on humans: Increased production of pro-inflammatory cytokines and Th1-like response in stress-induced anxiety. Cytokine. 1998;10(4):313–318. doi: 10.1006/cyto.1997.0290. [DOI] [PubMed] [Google Scholar]
- 13.Dinour LM, Bergen D, Yeh M-C. The food insecurity-obesity paradox: a review of the literature and the role food stamps may play. J Am Diet Assoc. 2007;107(11):1952–1961. doi: 10.1016/j.jada.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 14.Calder PC, Yaqoob P. Diet, Immunity and Inflammation. Woodhead Publishing Limited; Cambridge, UK: 2013. [Google Scholar]
- 15.Adam O, Beringer C, Kless T, et al. Anti-inflammatory effects of a low arachidonic acid diet and fish oil in patients with rheumatoid arthritis. Rheumatol Int. 2003;23(1):27–36. doi: 10.1007/s00296-002-0234-7. [DOI] [PubMed] [Google Scholar]
- 16.Prasad AS. Clinical, immunological, anti-inflammatory and antioxidant roles of zinc. Exp Gerontol. 2008;43(5):370–377. doi: 10.1016/j.exger.2007.10.013. [DOI] [PubMed] [Google Scholar]
- 17.García-Lafuente A, Guillamón E, Villares A, Rostagno MA, Martínez JA. Flavonoids as anti-inflammatory agents: implications in cancer and cardiovascular disease. Inflamm Res. 2009;58(9):537–552. doi: 10.1007/s00011-009-0037-3. [DOI] [PubMed] [Google Scholar]
- 18.Giugliano D, Esposito K. Mediterranean diet and metabolic diseases. Curr Opin Lipidol. 2008;19(1):63–68. doi: 10.1097/MOL.0b013e3282f2fa4d. [DOI] [PubMed] [Google Scholar]
- 19.Marques-Rocha JL, Milagro FI, Mansego ML, Zulet MA, Bressan J, Martínez JA. Expression of inflammation-related miRNAs in white blood cells from subjects with metabolic syndrome after 8 wk of following a Mediterranean diet–based weight loss program. Nutrition. 2016;32(1):48–55. doi: 10.1016/j.nut.2015.06.008. [DOI] [PubMed] [Google Scholar]
- 20.Hanson KL, Connor LM. Food insecurity and dietary quality in US adults and children: a systematic review. Am J Clin Nutr. 2014;100(2):684–692. doi: 10.3945/ajcn.114.084525. [DOI] [PubMed] [Google Scholar]
- 21.Cavicchia PP, Steck SE, Hurley TG, et al. A New Dietary Inflammatory Index Predicts Interval Changes in Serum High-Sensitivity C-Reactive Protein. J Nutr. 2009;139(12):2365–2372. doi: 10.3945/jn.109.114025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shivappa N, Steck SE, Hurley TG, Hussey JR, Hébert JR. Designing and developing a literature-derived, population-based dietary inflammatory index. Public Health Nutr. 2014;17(8):1689–1696. doi: 10.1017/S1368980013002115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rutishauser IH. Dietary intake measurements. Public Health Nutr. 2005;8(7a):1100–1107. doi: 10.1079/phn2005798. [DOI] [PubMed] [Google Scholar]
- 24.Shivappa N, Steck SE, Hurley TG, et al. A population-based dietary inflammatory index predicts levels of C-reactive protein in the Seasonal Variation of Blood Cholesterol Study (SEASONS) Public Health Nutr. 2014;17(8):1825–1833. doi: 10.1017/S1368980013002565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tabung FK, Steck SE, Zhang J, et al. Construct validation of the dietary inflammatory index among postmenopausal women. Ann Epidemiol. 2015;25(6):398–405. doi: 10.1016/j.annepidem.2015.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wirth MD, Shivappa N, Davis L, et al. Construct validation of the Dietary Inflammatory Index among African Americans. J Nutr Health Aging. 2017;21(5):487–491. doi: 10.1007/s12603-016-0775-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shivappa N, Wirth MD, Hurley TG, Hébert JR. Association between the dietary inflammatory index (DII) and telomere length and C-reactive protein from the National Health and Nutrition Examination Survey-1999–2002. Mol Nutr Food Res. 2017;61(4):1–7. doi: 10.1002/mnfr.201600630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lai JS, Hiles S, Bisquera A, Hure AJ, McEvoy M, Attia J. A systematic review and meta-analysis of dietary patterns and depression in community-dwelling adults. Am J Clin Nutr. 2014 Jan;99(1):181–197. doi: 10.3945/ajcn.113.069880. ajcn.069880. [DOI] [PubMed] [Google Scholar]
- 29.Hu FB. Dietary pattern analysis: a new direction in nutritional epidemiology. Curr Opin Lipidol. 2002;13(1):3–9. doi: 10.1097/00041433-200202000-00002. [DOI] [PubMed] [Google Scholar]
- 30.Shivappa N, Hébert JR, Rietzschel ER, et al. Associations between dietary inflammatory index and inflammatory markers in the Asklepios Study. Br J Nutr. 2015;113(04):665–671. doi: 10.1017/S000711451400395X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lucas M, Chocano-Bedoya P, Schulze MB, et al. Inflammatory dietary pattern and risk of depression among women. Brain Behav Immun. 2014;36:46–53. doi: 10.1016/j.bbi.2013.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sánchez-Villegas A, Ruíz-Canela M, de la Fuente-Arrillaga C, et al. Dietary inflammatory index, cardiometabolic conditions and depression in the Seguimiento Universidad de Navarra cohort study. Br J Nutr. 2015;114(09):1471–1479. doi: 10.1017/S0007114515003074. [DOI] [PubMed] [Google Scholar]
- 33.Shivappa N, Schoenaker DAJM, Hebert JR, Mishra GD. Association between inflammatory potential of diet and risk of depression in middle-aged women: the Australian Longitudinal Study on Women’s Health. Br J Nutr. 2016;116(6):1077–1086. doi: 10.1017/S0007114516002853. [DOI] [PubMed] [Google Scholar]
- 34.Akbaraly TN, Kerlau C, Wyart M, et al. Dietary Inflammatory Index and Recurrence of Depressive Symptoms Results from the Whitehall II Study. Clin Psychol Sci. 2016 Nov;4(6):1125–1134. doi: 10.1177/2167702616645777. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bergmans RS, Malecki KM. The association of dietary inflammatory potential with depression and mental well-being among US adults. Prev Med. 2017;99:313–319. doi: 10.1016/j.ypmed.2017.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Garcia-Arellano A, Ramallal R, Ruiz-Canela M, et al. Dietary Inflammatory Index and Incidence of Cardiovascular Disease in the PREDIMED Study. Nutrients. 2015;7(6):4124–4138. doi: 10.3390/nu7064124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wirth MD, Shivappa N, Hurley TG, Hébert JR. Association between previously diagnosed circulatory conditions and a dietary inflammatory index. Nutr Res. 2016;36(3):227–233. doi: 10.1016/j.nutres.2015.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sokol A, Wirth MD, Manczuk M, et al. Association between the dietary inflammatory index, waist-to-hip ratio and metabolic syndrome. Nutr Res. 2016;36(11):1298–1303. doi: 10.1016/j.nutres.2016.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tabung FK, Steck SE, Ma Y, et al. The association between dietary inflammatory index and risk of colorectal cancer among postmenopausal women: results from the Women’s Health Initiative. Cancer Causes Control. 2015;26(3):399–408. doi: 10.1007/s10552-014-0515-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shivappa N, Bosetti C, Zucchetto A, et al. Association between dietary inflammatory index and prostate cancer among Italian men. Br J Nutr. 2015;113(02):278–283. doi: 10.1017/S0007114514003572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tabung FK, Steck SE, Liese AD, et al. Association between dietary inflammatory potential and breast cancer incidence and death: results from the Women’s Health Initiative. Br J Cancer. 2016;114(11):1277–1285. doi: 10.1038/bjc.2016.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Olson CM. Food insecurity in women: A recipe for unhealthy trade-offs. Top Clin Nutr. 2005;20(4):321–328. [Google Scholar]
- 43.Lee JS, Frongillo EA., Jr Factors Associated with Food Insecurity Among U.S. Elderly Persons Importance of Functional Impairments. J Gerontol B Psychol Sci Soc Sci. 2001;56(2):S94–S99. doi: 10.1093/geronb/56.2.s94. [DOI] [PubMed] [Google Scholar]
- 44.Zipf G, Chiappa M, Porter KS, Ostchega Y, Lewis BG, Dostal J. National health and nutrition examination survey: plan and operations, 1999–2010. Vital Health Stat Ser 1 Programs Collect Proced. 2013;(56):1–37. [PubMed] [Google Scholar]
- 45.Levy CJ. Federal Poverty Level. In: Loue SJ, Sajatovic M, editors. Encyclopedia of Aging and Public Health. Springer US; New York, NY: 2008. pp. 356–358. [Google Scholar]
- 46.Bickel G, Nord M, Price C, Hamilton W, Cook J. Guide to Measuring Household Food Security. Office of Analysis, Nutrition, and Evaluation, Food and Nutrition Service, USDA; Alexandria, VA: 2000. Revised. [Google Scholar]
- 47.Cook JT, Black M, Chilton M, et al. Are Food Insecurity’s Health Impacts Underestimated in the U.S. Population? Marginal Food Security Also Predicts Adverse Health Outcomes in Young U.S. Children and Mothers. Adv Nutr Int Rev J. 2013;4(1):51–61. doi: 10.3945/an.112.003228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Miilunpalo S, Vuori I, Oja P, Pasanen M, Urponen H. Self-rated health status as a health measure: The predictive value of self-reported health status on the use of physician services and on mortality in the working-age population. J Clin Epidemiol. 1997;50(5):517–528. doi: 10.1016/s0895-4356(97)00045-0. [DOI] [PubMed] [Google Scholar]
- 49.Tarasuk V. Health implications of food insecurity. In: Raphael D, editor. Social Determinants of Health: Canadian Perspectives. 321ȓ342. Toronto, Canada: Canadian Scholars’ Press Inc; 2004. pp. 187–200. [Google Scholar]
- 50.SAS Software. Cary, NC, USA: SAS Institute Inc; 2013. [Google Scholar]
- 51.Willett W. Nutritional Epidemiology. Oxford University Press; 2012. [Google Scholar]
- 52.Jones AD. Food Insecurity and Mental Health Status: A Global Analysis of 149 Countries. Am J Prev Med. 2017;53(2):264–273. doi: 10.1016/j.amepre.2017.04.008. [DOI] [PubMed] [Google Scholar]
- 53.Gowda C, Hadley C, Aiello AE. The Association Between Food Insecurity and Inflammation in the US Adult Population. Am J Public Health. 2012;102(8):1579–1586. doi: 10.2105/AJPH.2011.300551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wirth M, Burch J, Shivappa N, et al. Dietary Inflammatory Index Scores Differ by Shiftwork Status: NHANES 2005–2010. J Occup Environ Med Am Coll Occup Environ Med. 2014;56(2):145–148. doi: 10.1097/JOM.0000000000000088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Leung CW, Epel ES, Ritchie LD, Crawford PB, Laraia BA. Food insecurity is inversely associated with diet quality of lower-income adults. J Acad Nutr Diet. 2014;114(12):1943–1953e2. doi: 10.1016/j.jand.2014.06.353. [DOI] [PubMed] [Google Scholar]
- 56.Laur CV, McNicholl T, Valaitis R, Keller HH. Malnutrition or frailty? Overlap and evidence gaps in the diagnosis and treatment of frailty and malnutrition. Appl Physiol Nutr Metab. 2017 Mar;45(5):449–458. 1–10. doi: 10.1139/apnm-2016-0652. [DOI] [PubMed] [Google Scholar]
- 57.Locher JL, Ritchie CS, Roth DL, Baker PS, Bodner EV, Allman RM. Social isolation, support, and capital and nutritional risk in an older sample: ethnic and gender differences. Soc Sci Med. 2005;60(4):747–761. doi: 10.1016/j.socscimed.2004.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Johansson G, Wikman A, Ahrén A-M, Hallmans G, Johansson I. Underreporting of energy intake in repeated 24-hour recalls related to gender, age, weight status, day of interview, educational level, reported food intake, smoking habits and area of living. Public Health Nutr. 2001;4(04):919–927. doi: 10.1079/phn2001124. [DOI] [PubMed] [Google Scholar]
- 59.De Keyzer W, Huybrechts I, De Vriendt V, et al. Repeated 24-hour recalls versus dietary records for estimating nutrient intakes in a national food consumption survey. Food Nutr Res. 2011;55:7307. doi: 10.3402/fnr.v55i0.7307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hutcheon JA, Chiolero A, Hanley JA. Random measurement error and regression dilution bias. BMJ. 2010;340:c2289. doi: 10.1136/bmj.c2289. [DOI] [PubMed] [Google Scholar]