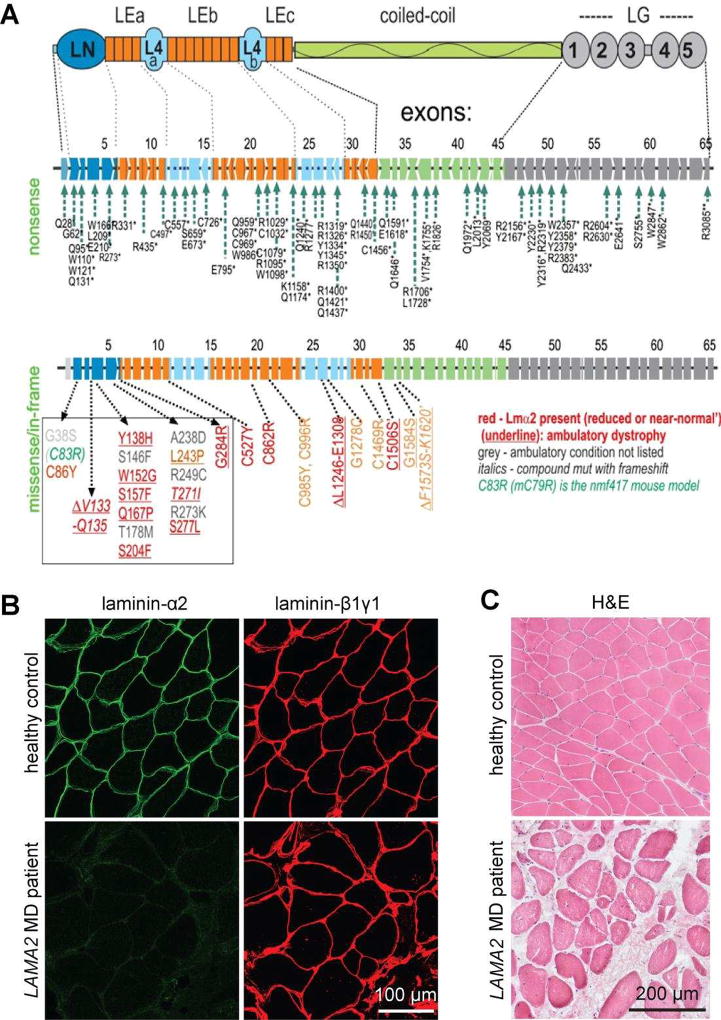
Figure 1. Mutations and muscle phenotype in LAMA2 MD.
A. Domain structure of laminin-α2 (top), location of mutations that cause the severe, non-ambulatory form (middle) or the ambulatory form of LAMA2 MD (bottom). The mutations of the severe form span the entire length of the gene and its 65 exons and intervening introns. They completely lack expression of the laminin-α2 resulting from premature chain-termination mutations. The ambulatory dystrophy is typically seen with missense mutations causing reduced and sometimes even normal expression of laminin-α2, resulting from amino acid substitutions or small deletions. Note that 17 of these mutations are clustered in the N-terminal LN domain that enables the laminin to polymerize. B. Cross-sections of muscle biopsies from normal and LAMA2 MD patients stained with antibodies directed to laminin-α2 or laminin-β1 and –γ1. Note that LAMA2 patient biopsies are negative for laminin-α2 but express similar amount of the laminin-β1 and -γ1. C. Haematoxylineosin stained muscle cross-sections from healthy controls and LAMA2 MD patients. Note the loss of muscle fibers and replacement with non-muscle tissue and the heterogeneous size of muscle fibers in LAMA2 MD patients compared to healthy controls.