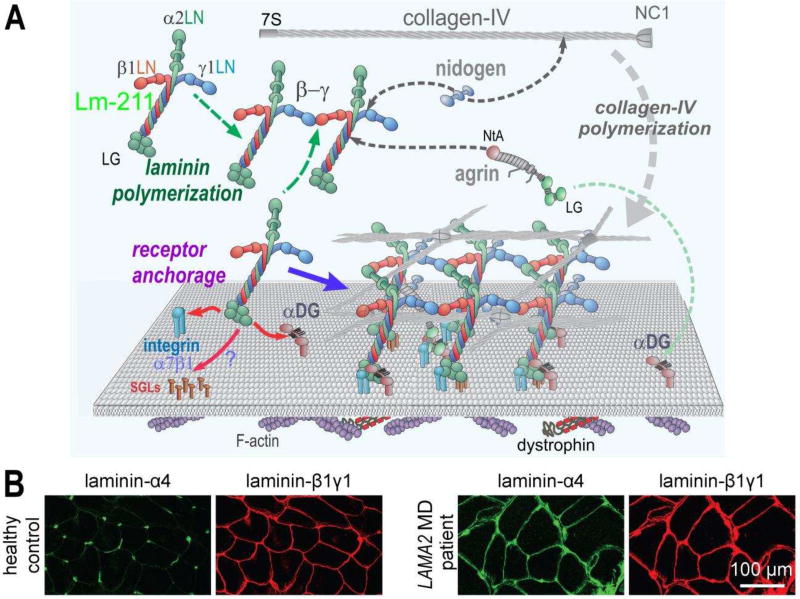
Figure 2. Normal and dystrophic basement membrane assembly and composition.
A. In skeletal muscle, Lm-211 forms the initial polymer nascent scaffolding by binding to integrin α7β1, αDG, and possibly also to sulfated glycolipids (SGL) via the LG domains. The α7β1 integrin and αDG form linkages through cellular adaptor proteins to actin and other cytoskeletal cables. Lm-21 1 also polymerizes through the three different LN domains that interact to form a ternary node. Nidogen-1 (and −2) binds to the laminin-γ1subunit (domain LEb3) and to collagen-IV, acting as a bridge, with the collagen polymerizing to form a second network. Agrin binds to the coiled-coil of the Lm-211 via its NtA domain and to αDG with the C-terminal LG domains, possibly acting as a collateral linker. All structural components become directly or indirectly tethered to cell receptors through the polymerizing laminin. Lm-411 is a component of the peripheral nerve Schwann cell basement membrane but absent in normal sarcolemmal basement membrane. B. Immuno-stained cross-section of skeletal muscle from a healthy control and a LAMA2 MD patient. In healthy controls, laminin-α4 is largely restricted to blood vessel and the neuromuscular junction. In LAMA2 MD patients, laminin-α4 is found in muscle basement membrane hereby substituting for laminin-α2.