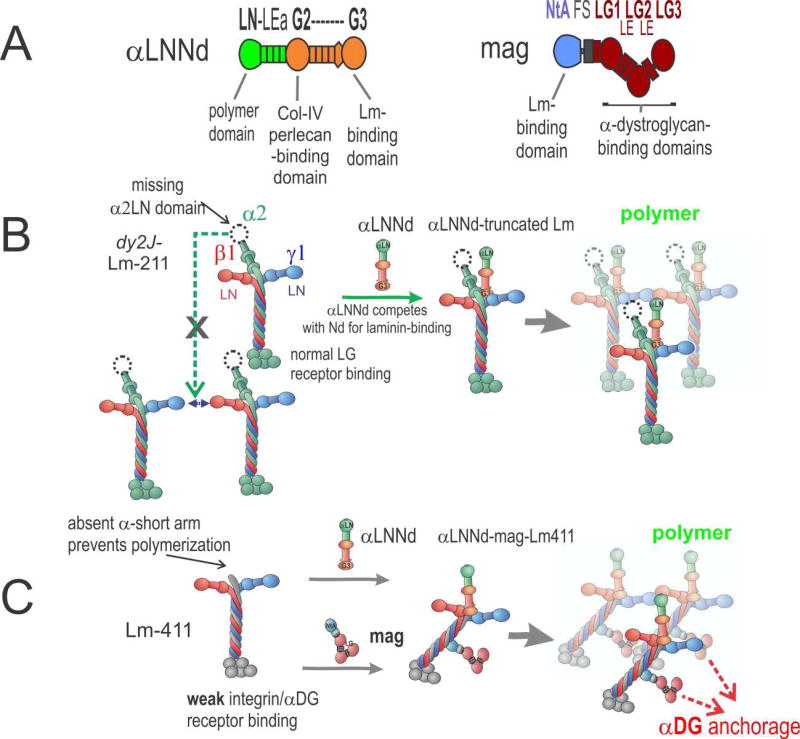
Figure 3. αLNNd and mag repair of laminin function.
A. Domain structure and functional activities of αLNNd and mag. Regions derived from laminin-α1 are in green; regions derived from nidogen-1 are in orange. Mag is a miniaturized version of agrin with N-terminal regions (blue) and C-terminal parts (red). B. In the ambulatory form of LAMA2 MD and its dy2J/dy2J mouse model, a truncated version of Lm-211 (“dy2J–Lm-211”) is expressed. αLNNd binds to the nidogen-binding site and creates an artificial short arm with a functional LN domain. This enables polymerization and promotes assembly of a stable basement membrane. C. In the absence of laminin-α2, which causes the severe non-ambulatory form of LAMA2 MD, the amount of Lm-41 1 is increased. Lm-411 is unable to polymerize and binds poorly to integrin α7β1 and to αDG. Co-expression of αLNNd and mag provide the necessary domains for polymerization and αDG anchorage. Together the two linker proteins restore laminin assembly and muscle binding, which results in strong amelioration of the severe muscular dystrophy in dyW/dyW mice.