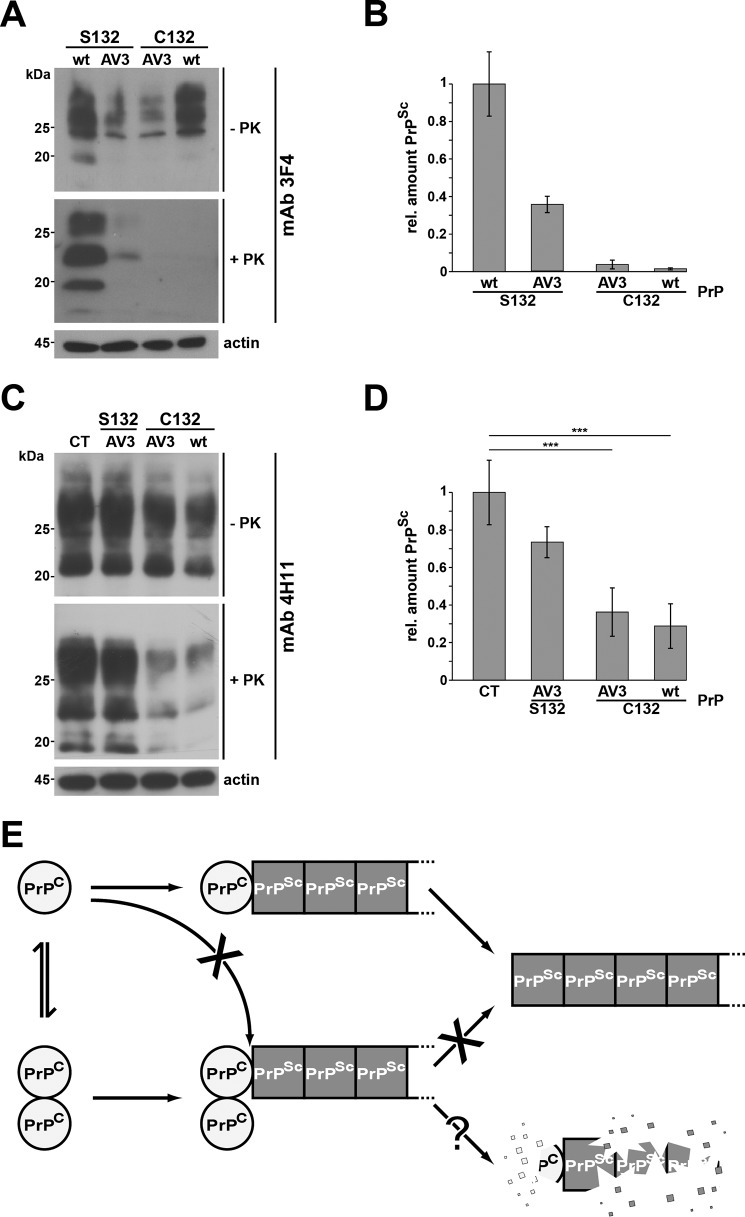
Figure 5.
Forced dimerization of PrPC interferes with propagation of PrPSc. A and C, persistently infected 22L-ScN2a cells were transiently transfected with the constructs indicated. Cell lysates were prepared and subjected to PK digestion (+PK) or left untreated (−PK) prior to immunoblot analysis using the monoclonal anti-PrP antibody 3F4 to exclusively detect the transfected PrP but not the endogenous PrPC (A) or using the monoclonal anti-PrP antibody 4H11 to detect endogenous mouse PrPC and PrPSc in addition (C). B and D, quantitative analysis of the amount of 3F4-positive (B) and total (D) PrPSc in transfected 22L-ScN2a cells. The relative (rel.) amount of PrP and PK-resistant PrPSc was measured densitometrically using ImageJ software. The relative amount of PK-resistant PrPSc present in cells expressing transfected WTPrPC (C) or control-transfected cells (D) was set as 1. Data represent mean ± S.D. of ≥3 independent experiments. E, putative model of the protective activity of PrPC dimers. Under physiological conditions, PrPC forms a dimer. Upon dissociation, PrPC monomers interact with and are converted by PrPSc. PrP dimers may interact with PrPSc, but conversion does not occur. In case PrPC dimers bind to PrPSc with a higher affinity than monomeric PrPC, interaction of monomeric PrPC with PrPSc is decreased, and its conversion is reduced. Alternatively or in addition, PrPSc in complex with PrPC dimers is subjected to increased intracellular degradation. Error bars represent S.D.