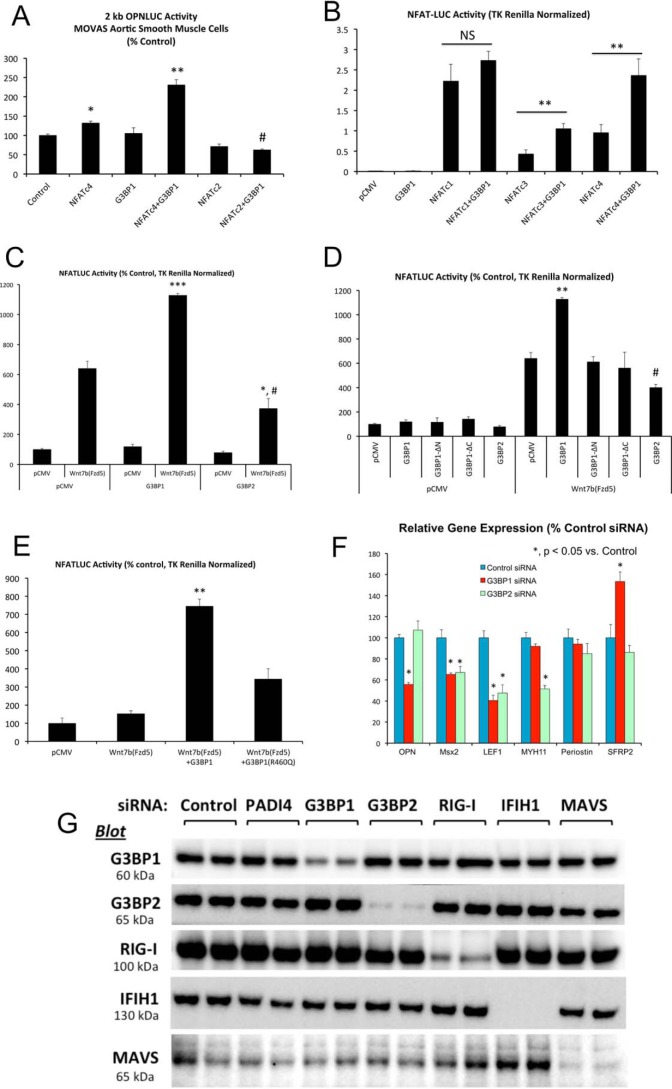
Figure 4.
G3BP1 enhances NFATc4-dependent transcription and supports OPN expression. A, MOVAS murine aortic VSM cells were transiently co-transfected with the 2-kb murine OPN promoter-luciferase reporter (OPNLUC) (96), with or without the pCMV-based expression vectors for NFATc4, NFATc2, and G3BP1 as indicated. TK Renilla was included as an internal control for transfection efficiency. Note that NFATc4 and G3BP1 synergistically increase OPN promoter activity. *, **, p ≤ 0.05 and p ≤ 0.01 versus control. #, p ≤ 0.01 versus G3BP1 + NFATc4. B, HEK293T cells were transiently transfected with a concatemerized NFAT cognate-driven luciferase reporter derived from the interleukin 2 promoter (NFATLUC) and the indicated expression vectors. Note that G3BP1 enhances both NFATc3- and NFATc4-dependent transcription. **, p ≤ 0.01 versus corresponding control. C, HEK293T cells were transiently transfected NFATLUC and pCMV-based expression vector for Wnt7b, Fzd5, G3BP1, and G3BP2 as indicated. Note that although G3BP1 enhances NFATLUC activation by the noncanonical Wnt7b/Fzd5 cascade, G3BP2 is inactive in this assay *, ***, p ≤ 0.05 and p ≤ 0.001 versus Wnt7b/Fzd5 with pCMV; #, p ≤ 0.001 versus G3BP1 with Wnt7b/Fzd5. D, both the NTF2 and C-terminal Arg methylation domains of G3BP1 are required to enhance Wnt/NFAT signaling. **, p ≤ 0.01 versus all others; #, p ≤ 0.05 versus Wnt7b/Fzd5 with pCMV, and p ≤ 0.01 versus Wnt7b/Fzd5 with G3BP1. E, G3BP1(R460Q) substitution in human G3BP1 reduces G3BP1-dependent enhancement of noncanonical Wnt/NFAT signaling. **, p ≤ 0.01 versus all others. F, RNAi targeting G3BP1 down-regulates OPN mRNA accumulation in primary aortic VSM. G3BP2 siRNA does not inhibit OPN expression, even though it significantly reduces Msx2, LEF1, and Myh11 (*, p ≤ 0.05 versus control siRNA). G, Western blotting analyses demonstrate efficient and selective reductions in G3BP1 and G3BP2 proteins by RNAi in aortic VSM cultures.