Figure 2.
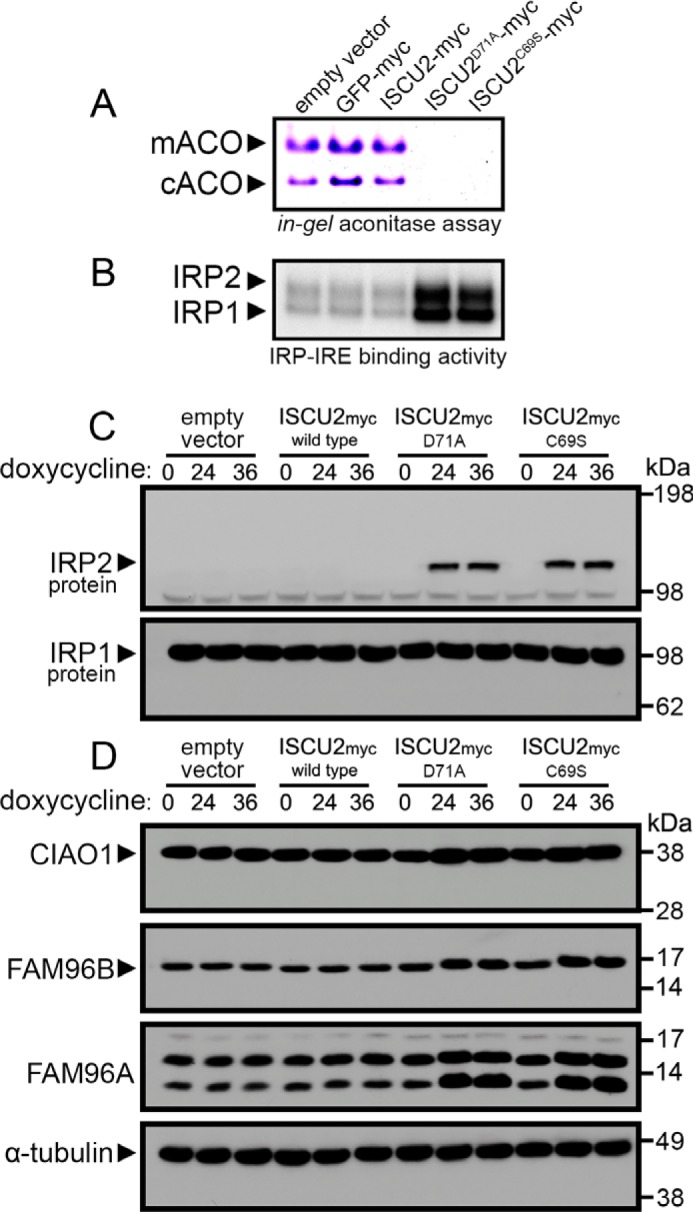
Loss of aconitase activity and activation of iron-regulatory proteins in cells expressing dominant–negative ISCU mutants. A, aconitase activity gel demonstrated decreased mitochondrial and cytosolic aconitase activities in cells expressing ISCU2D71A or ISCU2C69S following a 24-h incubation with doxycycline. B, analysis of ferritin IRE binding activity of IRP1 and IRP2 by an electrophoretic mobility shift assay demonstrated increased IRP activity in cells expressing ISCU2D71A or ISCU2C69S. C, immunoblot analyses of protein levels in cells expressing ISCU2D71A or ISCU2C69S for either 24 or 36 h demonstrated robust stabilization of IRP2 protein in cells expressing ISCU2D71A or ISCU2C69S, whereas IRP1 protein abundance was unchanged. D, protein abundance of CIAO1, FAM96B, and FAM96A were also evaluated by immunoblot. α-tubulin was the loading control for cytosolic proteins.
